CO2TransportClassNotes
advertisement

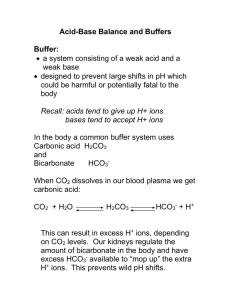
Carbon Dioxide Transport and Acid-Base Balance Understanding carbon dioxide transport is essential to the study of pulmonary physiology as well as to the interpretation of arterial blood gases in the clinical setting. There are three main areas that will be discussed. Carbon dioxide transport from the tissues to the lungs Acid-base balance The relationship of the partial pressure of carbon dioxide (PCO2), bicarbonate (HCO3-), and pH in acid-base balance CARBON DIOXIDE TRANSPORT At rest, normal tissue cell metabolism produces about 200 mL of carbon dioxide per minute. This gas is transported from the tissue cells to the lungs by the following mechanisms. In Plasma Carbamino compound o Carbon dioxide dissolves in plasma and chemically combines with protein molecules to form a carbamino compound. o This accounts for about 1% of all carbon dioxide that dissolves in the plasma. Bicarbonate o Carbon dioxide combines with water (hydrolysis) to form carbonic acid (H2CO3), which rapidly ionizes into bicarbonate HCO3- and hydrogen ions H+. o This mechanism accounts for about 5% of the carbon dioxide that dissolves in the plasma. Dissolved carbon dioxide o About 5% of the total carbon dioxide released at the lungs is dissolved carbon dioxide in the plasma. o This portion of the carbon dioxide transported in the blood is measured to assess the partial pressure of carbon dioxide (PCO2). o The concentration of carbonic acid (H2CO3) is proportional (about 1/1000) to the partial pressure of the carbon dioxide. H2CO3 = PCO2 x 0.03 For example if PCO2 is 40 mm Hg, then: H2CO3 = 40 x 0.03 = 1.2 mEq/L In Red Blood Cell Dissolved carbon dioxide o Carbon dioxide dissolves in the intracellular fluid of red blood cells. o This accounts for about 5% of the total carbon dioxide released at the lungs. Carbamino-Hb o The compound formed when the carbon dioxide combines with hemoglobin. o This reaction releases oxygen, which becomes available for tissue metabolism. o This accounts for about 21% of the carbon dioxide carried from the tissues. Bicarbonate o About 63% of the carbon dioxide is transported to tissue cells in the form of bicarbonate (HCO3-). o In plasma, the normal ratio of bicarbonate (HCO3-) to carbonic acid (H2CO3) is 20:1. This ratio keeps the blood pH level within the normal range of 7.35 to 7.45. Blood pH becomes more alkaline as the ratio increases (for example, 22:1). Blood pH becomes less alkaline as the ratio decreases (for example,18:1). o Refer to Figure 7-1 in the text for an illustration of how carbon dioxide is converted to bicarbonate at the tissue sites. CARBON DIOXIDE ELIMINATION AT THE LUNGS As venous blood enters the alveolar capillaries; the chemical reactions occurring at the tissue level are reversed and continue until the carbon dioxide pressure is equal throughout the system. Refer to Figure 7-2 in the text for an illustration of how bicarbonate is transformed back to carbon dioxide in the alveoli. See Table 7-1 in the text for a summary of the percentage and quantity of total carbon dioxide that is transported every minute by each of the six mechanisms described above. CARBON DIOXIDE DISSOCIATION CURVE The loading and unloading of carbon dioxide in the blood can be illustrated graphically using the carbon dioxide dissociation curve. Compared with the oxygen dissociation curve, the carbon dioxide dissociation curve shows a more direct relationship between the partial pressure of carbon dioxide (PCO2) and the carbon dioxide content in the blood. o The shape of the curve is more linear. The level of saturation of hemoglobin with oxygen also affects the carbon dioxide dissociation curve. o When the hemoglobin saturation is high, there is less carbon dioxide content for any given partial pressure (PCO2). Haldane Effect o Deoxygenated hemoglobin enhances the loading of carbon dioxide at the tissue sites. o Increased levels of oxyhemoglobin enhance the unloading of carbon dioxide at the lungs. Figures 7-3 and 7-4 in the text provide examples of a carbon dioxide dissociation curve. ACID-BASE BALANCE An understanding of the following terms is essential for understanding acid-base balance. Electrolytes: ions that can conduct a current in solution Buffer: substances that can neutralize acids and bases to prevent a significant change in pH Strong acid: an acid that dissociates completely into hydrogen ions (H+) and an anion (hydrogen ion donor) Weak acid: an acid that dissociates only partially into ions Strong base: a base that dissociates completely into hydroxyl ions (OH-) Weak base: a base that reacts with water to form OH- in an equilibrium Dissociation constant: weak acid or base systems that have an equilibrium between the molecular form and its ions o HA ↔ [H+] + [A-] HA is the molecular form [H+] and [A-] are the ions o Ka = [H+] + [A-] / [HA] The pH Scale The pH measurement is important because hydrogen ion concentration will affect the metabolic function of the body’s cells. A pH of 7 is neutral, below 7 is acidic, and above 7 is basic. The pH is defined as the negative logarithm, to the base 10, of the H+ concentration. o pH = -log10[H+] The normal pH of arterial blood is 7.35 to 7.45. o An acid increases the hydrogen ion concentration of a solution and causes the pH value to decrease. o A base decreases the hydrogen ion concentration and causes the pH value to increase. The normal narrow pH range is maintained by: o The buffer systems of the blood and tissues o The respiratory system’s ability to regulate the elimination of carbon dioxide o The renal system’s ability to regulate the excretion of hydrogen and the reabsorption of bicarbonate ions. The Buffer Systems Buffer action is the ability of an acid-base mixture to resist large changes in pH. The most significant buffer combinations in the body are as follows: o Plasma Carbonic acid/sodium bicarbonate Sodium acid phosphate/sodium alkaline phosphate Acid proteinate/sodium proteinate o Erythrocytes Acid hemoglobin/potassium hemoglobin Potassium acid phosphate/potassium alkaline phosphate The carbonic acid/sodium bicarbonate combination (H2CO3/NaHCO3) is the most important buffer combination for maintaining acid/base balance in the blood. o Example: When a strong acid such as hydrochloric acid (HCl) is added to a H2CO3/NaHCO3 system, the following reaction occurs: HCl + NaHCO3- ↔ H2CO3 + NaCl This reaction reduces the strong acid (HCl) into a weak acid (H2CO3) and a neutral salt (NaCl). The decrease in pH toward the acidic range is minimal. o Example: When a strong base such as sodium hydroxide (NaOH) is added to a H2CO3/NaHCO3 system, the following reaction occurs: NaOH + H2CO3 ↔ NaHCO3 + H2O This reaction reduces the strong base (NaOH) into a weak base (NaHCO3) and water (H2O). The increase in pH toward the basic range is minimal. The Henderson-Hasselbalch Equation The Henderson-Hasselbalch equation allows calculation of the pH of the blood. Calculation o pH = pK + log [HCO3-] / [H2CO3] pK is the dissociation constant of the acid portion (H2CO3) of the buffer combination normally, the pK is 6.1 o pH = 6.1 + log [24 mEq/L / 1.2 mEq/L] o pH = 6.1 + log 20 / 1 o pH = 6.1 + 1.3 o pH = 7.4 The major component of the Henderson-Hasselbalch equation is the ratio of HCO3- to H2CO3, which is normally 20:1 (24 mEq/L / 1.2 mEq/L) o When the ratio increases, the pH increases: pH = 6.1 + log 25 / 1 pH = 6.1 + 1.4 pH = 7.5 o When the ratio decreases, the pH decreases: pH = 6.1 + log 15 / 1 pH = 6.1 + 1.18 pH = 7.29 THE ROLE OF THE PCO2/HCO3-/pH RELATIONSHIP IN ACID-BASE BALANCE RESPIRATORY ACID-BASE IMBALANCES Most carbon dioxide is transported from the tissues to the lungs as bicarbonate (HCO3-). o As the carbon dioxide level increases, the plasma partial pressure of carbon dioxide (PCO2), bicarbonate (HCO3-), and carbonic acid (H2CO3) increase. o As the carbon dioxide level decreases, the plasma partial pressure of carbon dioxide (PCO2), bicarbonate (HCO3-), and carbonic acid (H2CO3) decrease. Because blood pH depends on the ratio between the plasma bicarbonate (base) and the plasma carbonic acid (acid), acute ventilatory changes will immediately affect the pH. Acute changes in the carbonic acid (H2CO3) level play a much more powerful role in altering pH than acute changes in bicarbonate (HCO3-) do. o For every carbonic acid (H2CO3) molecule increase or decrease, there must be a 20-bicarbonate (HCO3-) molecule increase or decrease, respectively, to maintain the normal 20:1 ratio between HCO3- and H2CO3. o Respiratory changes in acid-base status are evident almost immediately; renal changes to compensate for respiratory alterations in acid-base balance take longer. Acute Ventilatory Failure Acute ventilatory failure is acute hypoventilation as a result of acute pulmonary disease, respiratory center depression or respiratory pump failure. During acute ventilatory failure: PACO2 increases. PCO2, HCO3-, and H2CO3 levels increase. Decreased HCO3- to H2CO3 ratio develops. Blood pH decreases (becomes more acidic). The figure below is a nomogram of the PCO2, HCO3-, and H2CO3 relationship. The PCO2/HCO3-/pH nomogram is a useful tool for confirming the presence of: Respiratory acid-base imbalances Metabolic acid-base imbalances Combined metabolic and respiratory acid-base imbalances Chronic Ventilatory Failure and Renal Compensation If hypoventilation (increased PaCO2) lasts for more than 24 to 48 hours, the kidneys will work to compensate for decreased pH by retaining HCO3- in the blood. Renal compensation can be verified when the calculated HCO3- and pH readings are higher than expected for a particular PCO2 level. o If the PCO2 level is about 80 mm Hg, the pH should be less than 7.2 and the HCO3- level should be about 30 mEq/L. o If HCO3- and pH levels are higher than these values, partial renal compensation (retention of HCO3-) has occurred. When the HCO3- level increases enough to return the acidic pH to the normal range of 7.35 to 7.45, complete renal compensation has occurred. Refer to Figure 7-9 in the text. Note: As a rule, the kidneys do not overcompensate for an abnormal pH. However, an exception exists for persons with chronic hypoventilation (as with COPD). In these patients, it is not unusual to find a pH greater than 7.4 due to other chemical interactions between electrolytes and fluid compartments in the body. Acute Alveolar Hyperventilation Acute alveolar hyperventilation is acute hyperventilation as a result of acute pulmonary disease; respiratory center injury or illness; pain or anxiety. During acute hyperventilation: PACO2 decreases. PCO2, HCO3-, and H2CO3 levels decrease. An increased HCO3- to H2CO3 ratio develops. Blood pH increases (becomes more alkaline). Refer to Figure 7-8 in the text. Chronic Alveolar Hyperventilation and Renal Compensation If hyperventilation lasts for more than 24 to 48 hours, the kidneys attempt to correct the increased pH by excreting excess HCO3- in the urine. Renal compensation can be verified when calculated HCO3- and pH readings are lower than expected for a particular PCO2 level. o If PCO2 is 22 mm Hg, the pH level should be greater than 7.5 and HCO3- levels should be about 19 mEq/L. If the HCO3- and pH levels are lower than these values, partial renal compensation has occurred. If the HCO3 level decreases enough to return the alkalotic pH to the normal range of 7.35 to 7.45, complete renal compensation has occurred. Refer to Figure 7-11 in the text. METABOLIC ACID-BASE IMBALANCES Metabolic Acidosis Metabolic acidosis is the presence of other acids not related to increase in PCO2 or to renal compensation. The calculated HCO3- and pH will be lower than expected for a particular PCO2 level. o For example, a HCO3- reading of 17 mEq/L and a pH of 7.25 would be less than expected in a patient who has a PCO2 of 40 mm Hg. Figure 7-12 in the text illustrates this. Common causes of metabolic acidosis include: o Lactic acidosis The end product of anaerobic biochemical reactions activated when the oxygen level is inadequate to meet tissue needs. o Ketoacidosis The end product of the metabolic process that produces ketones in response to low blood insulin (resulting in very high blood sugar) in people with diabetes. o Renal failure Results in the accumulation of hydrogen ions. Treatment o Correct the underlying problem. o Severe metabolic acidosis is treated with careful administration of sodium bicarbonate (NaHCO3). Chronic Metabolic Acidosis and Respiratory Compensation Increased ventilatory rate and depth (tachypnea and hyperpnea) is an immediate compensating response to metabolic acidosis. The PaCO2 decreases, the hydrogen ion concentration decreases, and the metabolic acidosis is compensated. Complete respiratory compensation occurs when PaCO2 decreases enough to move pH levels back to the normal range of 7.35 to 7.45. Refer to Figure 7-12 in the text. Metabolic Alkalosis Metabolic alkalosis is characterized by the presence of other bases not related to a decreased PCO2 level or to renal compensation. Calculated HCO3- and pH readings will be higher than expected for a particular PCO2 level. Common causes of metabolic alkalosis include the following: o Hypokalemia The depletion of total body potassium as a result of: prolonged IV therapy without adequate potassium replacement diuretic therapy diarrhea Symptoms of hypokalemia are metabolic alkalosis, muscle weakness, and cardiac dysrhythmia. o Hypochloremia Occurs when the chloride ion concentration decreases and bicarbonate ions increase in an attempt to maintain balance in the blood serum (since both are negative ions). As bicarbonate increases, the blood serum becomes alkalotic. o Gastric suction or vomiting Causes a loss of hydrochloric acid, which produces a relative increase in blood serum base. o Excessive administration of corticosteroids Causes the kidneys to excrete hydrogen ions and potassium o Excessive administration of sodium bicarbonate Used to treat metabolic acidosis. Treatment o Correct the underlying electrolyte problem. o Administer ammonium chloride (NH4Cl). Refer to Figure 7-13 in the text. Chronic Metabolic Alkalosis and Respiratory Compensation The ventilatory rate and depth decrease in response to metabolic alkalosis, causing PaCO2 to increase. Increased hydrogen ions produced by elevated blood PaCO2 compensate for the metabolic alkalosis. When the PaCO2 increases enough to move the pH back to the normal range of 7.35 to 7.45, complete respiratory compensation has occurred. Refer to Figure 7-13 in the text. BASE EXCESS / DEFICIT The PCO2 / HCO3- / pH nomogram also serves as an excellent tool to calculate the patient’s total base excess / deficit. Allows non-respiratory acid-base imbalances to be quantified. Reported in milliequivalents per liter (mEq/L) of base above or below the normal buffer base line of the PCO2 / HCO3- / pH nomogram. For example, if the pH is 7.25 and the HCO3- is 17 mEq/L, the nomogram will confirm the presence of o Metabolic acidosis o Commonly reported as a base excess (BE) of -7 mEq/L. The “negative” sign indicates that it is a deficit. In contrast, if the pH is 7.5 and the HCO3- is 31 mEq/L, then the nomogram will confirm the presence of o Metabolic alkalosis o Commonly reported as a base excess (BE) of 7 mEq/L.