Limit of solubility reported
advertisement

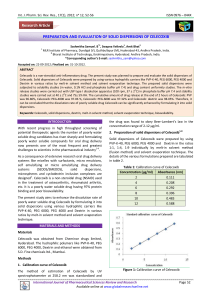
Limit of solubility Solution used References Water Temperature °C RT 2.55 ± 0.12 μg/ml = 6.68 μM 3 to 7 μg/ml= 7.9 to 18.35 μM Aqueous solution pH 7 40°C [2,3,4] 6 μg/mL = 15.7 μM Glycine/NaOH pH 7.6 25°C [5] 7 μg/mL = 18.4 μM Water 14 μg/mL = 33 μM Glycine/NaOH pH 9.2 0.015 μg/mL = 39 μM 80% Water / 20% ethanol About 1 μg/mL = 2.6 μM Water, NR [6] About 5 μg/mL= 13.1 μM Aqueous solutions pH<9, 5 to 40°C [7] 3.3 mg/L= 8,7 μM Water 25 [8-9] 5.03 mg/L = 13.2 μM Water (predicted solubility) Between 10 and 12.5 μM Water Between 5 and 7.5 μM Cell culture media* pH 7.3-7.4 Between 7.5 and 10 μM Cell culture media* pH 7.3-7.4, 0.5% serum Between 17.5 and 20 μM Cell culture media* pH 7.3-7.4, 10% serum Between 18 and 22 μM Cell culture media* pH 9.2, 0.5% serum Between 5 and 7.5μM Cell culture media* pH 6, 0.5% serum Between 100 and 160 μM Pure serum (FCS) > 18 μM Cell culture media*, 0.5 % serum, 10% [1] [8-10] 33 to 37°C Present work solvents** > 40 μM Cell culture media, 0.5 % serum, 20% solvents** Table S1. Comparison of Celecoxib solubility reported in the literature and determined in the present work. When the solubility is calculated by diluting Celecoxib from a DMSO stock, the final concentration of DMSO should be taken into account (0.2% in the present work, up to 1% in other studies). *Common cell culture media and solutions tested are DMEM, RPMI, RPMI/DMEM, HBSS, PBS. The presence/absence of NaHCO3, Phenol Red; Hepes does not make a significant difference. ** Solvents tested are DMSO, Ethanol, Methanol, Aceton, Glycerol. Besides increasing the solubility limit, these solvent visibly reduced number and dimension of precipitates/crystals at the concentrations of Celecoxib used in the toxicity assays. References 1. Gupta VR, Mutalik S, Patel MM, Jani GK (2007) Spherical crystals of celecoxib to improve solubility, dissolution rate and micromeritic properties. Acta Pharm 57: 173-184. 2. Subramanian N, Ghosal SK, Moulik SP (2004) Topical delivery of celecoxib using microemulsion. Acta Pol Pharm 61: 335-341. 3. Sinha VR, Anitha R, Ghosh S, Amita, Kumria R, et al. (2007) Physicochemical characterization and in vitro dissolution behaviour of celecoxib -beta-cyclodextrin inclusion complexes. Acta Pharm 57: 47-60. 4. Paulson SK, Vaughn MB, Jessen SM, Lawal Y, Gresk CJ, et al. (2001) Pharmacokinetics of celecoxib after oral administration in dogs and humans: effect of food and site of absorption. J Pharmacol Exp Ther 297: 638-645. 5. Seedher N, Bhatia S (2003) Solubility enhancement of Cox-2 inhibitors using various solvent systems. AAPS PharmSciTech 4: E33. 6. Guzman HR, Tawa M, Zhang Z, Ratanabanangkoon P, Shaw P (2007) Combined use of crystalline salt forms and precipitation inhibitors to improve oral absorption of celecoxib from solid oral formulations. J Pharm Sci 96: 2686-2702. 7. Datasheet Celebrex (www.celebrex.com) 8. Drugbank. Drug card for Celecoxib (DB00482) (http://www.drugbank.ca/drugs/DB00482) 9. SRC PhysProp Database (http://www.syrres.com/esc/physdemo.htm). Celecoxib , CAS Registry Number 169590-42-5 10. ALOGPS 2.1 program (http://www.vcclab.org/web/alogps/)