VSEPR
advertisement

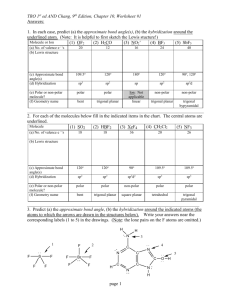
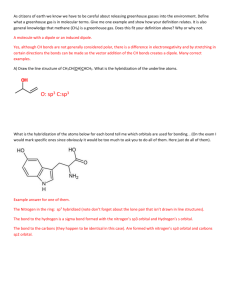
VSEPR Name__________________________ All of the following are covalent molecules or ions. Draw Lewis dot structures and use VSEPR to predict the shape of each. Use the gumdrops and toothpicks provided to build each chemical species. Be sure that you use one toothpick for each pair of electrons on the central atom. Under each one write the hybridization of the orbitals. 1. CO2 2. BF4- 3. H2CO Shape_____________ Shape_____________ Shape_____________ Hybridization_____________ Hybridization_____________ Hybridization_____________ 4. PCl5 5. SiH4 6. SeF6 Shape_____________ Shape_____________ Shape_____________ Hybridization_____________ Hybridization_____________ Hybridization_____________ 7. IF4- 8. F2CO 9. XeF4 Shape_____________ Shape_____________ Shape_____________ Hybridization_____________ Hybridization_____________ Hybridization_____________ 10. NO2- 11. O3 12. ClO3- Shape_____________ Shape_____________ Shape_____________ Hybridization_____________ Hybridization_____________ Hybridization_____________ 13. I3- 14. IOF5 (I is the central atom) 15. NH2- hape_____________ Shape_____________ Hybridization_____________ Hybridization_____________ Shape_____________ Hybridization_____________ S VSEPR (Valence Shell Electron Pair Repulsion) Regions of electron density 2 3 Representative Example formula Shape Hybridization MX2 MX3 CO2 BF3 sp sp2 3 4 MX2E MX4 SO2 CH4 4 MX3E NH3 4 5 MX2E2 MX5 H2O PF5 5 5 5 MX4E MX3E2 MX2E3 SF4 ICl3 I3- Linear (180o) Trigonal planar (120o) Bent (118o) Tetrahedral (109.5o) Trigonal pyramidal (107o) Bent (105o) Trigonal bipyramidal (TBP) See Saw T-shaped Linear 6 MX6 SCl6 Octahedral sp3d2 6 MX5E XeF5+ 6 MX4E2 ICl4- Square sp3d2 pyramidal Square planar sp3d2 sp2 sp3 sp3 sp3 sp3d sp3d sp3d sp3d Instructors note: Each pair of students needs to be provided with 12 gumdrops (2 of one color and 10 of another), a handful of toothpicks, and a paper napkin or towel to work on. Answers: 1. linear, sp 2. tetrahedral, sp3 3. trigonal planar, sp2 4. trigonal bipyramidal, sp3d 5. tetrahedral, sp3 6. octahedral, sp3d2 7. see saw, sp3d 8. trigonal planar, sp2 9. square planar, sp3d2 10. bent, sp2 11. bent, sp2 12. trigonal pyramidal, sp3 13. linear, sp3d 14. octahedral, sp3d2 15. bent, sp3 Extension: In structure 14, iodine was the central atom. Could oxygen be the central atom? Explain your answer. © 2004 by the Texas Education Agency Copyright © Notice. The Materials are copyrighted © and trademarked ™ as the property of the Texas Education Agency (TEA) and may not be reproduced without the express written permission of TEA, except under the following conditions: 1) Texas public school districts, charter schools, and Education Service Centers may reproduce and use copies of the Materials and Related Materials for the districts’ and schools’ educational use without obtaining permission from TEA. 2) Residents of the state of Texas may reproduce and use copies of the Materials and Related Materials for individual personal use only, without obtaining written permission of TEA. 3) Any portion reproduced must be reproduced in its entirety and remain unedited, unaltered and unchanged in any way. 4) No monetary charge can be made for the reproduced materials or any document containing them; however, a reasonable charge to cover only the cost of reproduction and distribution may be charged. Private entities or persons located in Texas that are not Texas public school districts, Texas Education Service Centers, or Texas charter schools or any entity, whether public or private, educational or non-educational, located outside the state of Texas MUST obtain written approval from TEA and will be required to enter into a license agreement that may involve the payment of a licensing fee or a royalty. For information contact: Office of Copyrights, Trademarks, License Agreements, and Royalties, Texas Education Agency, 1701 N. Congress Ave., Austin, TX 78701-1494; phone 512-463-7004; email: copyrights@tea.state.tx.us.