Retrosyn_Anal
advertisement

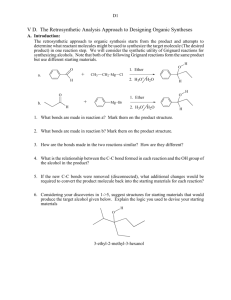
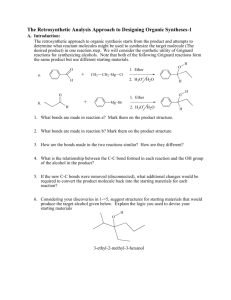
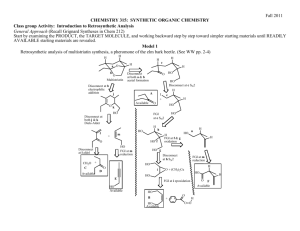
The Retrosynthetic Analysis Approach to Designing Organic Syntheses-3 D. Oxidation of Alcohols to Ketones 1. Introduction: In the previous section we learned how to use C-C disconnections, FGI’s of Grignard reagents and FGI dehydrations to synthesize larger alkenes and alcohols from smaller ketones or aldehydes and organic halides. This unit introduces methods for synthesizing ketones from alcohols, another FGI, since these reactions change functional groups, but do not alter the carbon structure of the reactant. The addition of this reaction to our synthetic repertoire greatly increases our abilities to build larger molecules from a number of smaller molecules. 2. Oxidation of 2˚ Alcohols to Ketones: Chromium Trioxide (CrO3) in acidic aqueous solution (H2SO4) is a common reagent for oxidation of alcohols. This metal oxide reagent is a relatively strong oxidizing reagent. It smoothly converts 2˚ alcohols to ketones. O HO H H2 SO4 + CrO3 H OH O H2 SO4 + CrO3 3. FGI-oxidation: The following FGI Retrosynthetic step indicates that the ketone can be synthesized from the corresponding alcohol by oxidation. O H OH FGI oxidation Although this is a simple addition to our reagent list, this oxidation increases our abilities to synthesize complex molecules from relatively simple ones. Consider the potential synthesis of the complex molecule shown below. One Grignard C-C disconnection reveals that the target molecule can be synthesized from phenyl magnesium chloride and 2,5-dimethyl-4heptanone. Cl Mg C-C disconnection O Grignard O + H With only the Grignard C-C disconnection, we were able to disconnect the carbon structure of an alcohol only once. Retrosynthetic Analysis-3 2 However, we now see that the ketone precursor revealed by the above disconnection can be synthesized from a 2˚ alcohol as indicated on page 80 and reproduced below: O H OH FGI oxidation This FGI reveals a compound that can be further disconnected with a Grignard C-C to reveal even simpler synthetic precursors. H OH O C-C disconnection Grignard Mg + Br H If we recall that Grignard Reagents can be synthesized from alkyl halides, we can look at the entire synthetic pathway as a set of disconnections and FGI's. The starting materials are enclosed in rectangles. Mg Cl O C-C disconnection O + Grignard H FGI-M FGI Oxidation O H OH H C-C disconnection + + Mgo Grignard Mg Cl Br FGI-M Br + Mgo Retrosynthetic Analysis-3 3 4. Out of Class Application: For our next lab discussion period use retrosynthetic analysis to devise syntheses for the following compounds from monosubstituted aromatic compounds and non-aromatic compounds with four or fewer carbon atoms. OH O Then write complete synthetic paths, including reagents and reaction conditions based on your retrosynthetic analyses. Be prepared to discuss both the retroanalysis and the synthesis.