STGCL.SWP.71.1_Plasmid DNA Transfection of cells_APR2010
advertisement

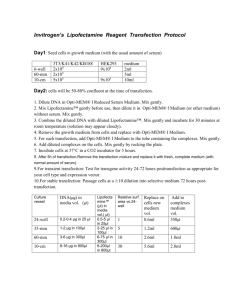
OHS026 Safe Work Procedure Faculty/Division Medicine Document number STGCL.SWP.71.1 School/ Divisional Unit St George Clinical School Initial Issue date 29/04/10 Current version 1.0 Current Version Issue date 29/04/10 Next review date 29/04/2012 The Writing Safe Work Procedures Guideline (OHS027) should be consulted to assist in the completion of this form. Safe Work Procedure Title and basic description Title: Plasmid DNA Transfection of mammalian cells in culture using Lipofectamine™ 2000, Lipfectamine Reagent and Lipofectamine Plus Reagent Description: To introduce nucleic acids into eukaryotic cells for expression of a particular mammalian gene. Associated risk assessment title and location: RA Plasmid DNA Transfection of mammalian cells Describe the activity or process Transfection of cells in culture typically involves opening transient pores or "holes" in the cells, to allow the uptake of material. Transfection can be carried out using calcium phosphate, by electroporation, or by mixing a cationic lipid such as lipofectamine Plus Reagent or lipofectamine 2000. The material produces liposomes, which fuse with the cell membrane and deposit their cargo inside. Procedure: 1. Adherent cells: One day before transfection, plate 0.5-2 x 105 cells in 500 µl of growth medium without antibiotics (in a 24 well plate) so that cells will be 90-95% confluent at the time of transfection. Suspension cells: Just prior to preparing complexes, plate 4-8 x 105 cells in 500 µl of growth medium without antibiotics (in a 24 well plate). 1. 24 hr after seeding, transfection is conducted with the following volumes: Plate Surface area per well 24 well 2 cm2 Volume of plating medium 500 µl DNA Transfection DNA lipofectamine 2000 0.8 µg (in 50µl SFM) 2ul (in 50µl SFM) 2. For each transfection sample, prepare complexes as follows: a. Dilute DNA in 50 µl of Opti-MEM® I Reduced Serum Medium without serum (or other medium without serum). Mix gently. b. Mix Lipofectamine™ 2000 gently before use, then dilute the appropriate amount in 50 µl of Opti-MEM® I Medium. Incubate for 5 minutes at room temperature (RT). Note: Proceed to Step c within 25 minutes. c. After the 5 minute incubation, combine the diluted DNA with diluted Lipofectamine™ 2000 (total volume = 100 µl). Mix gently and incubate for 20 minutes at RT (solution may appear cloudy). Note: Complexes are stable for 6 hours at RT. 3. Add the 100 µl of complexes to each well containing cells and medium. Mix gently by rocking the plate back and forth. 4. Incubate cells at 37°C in a CO2 incubator for 18-48 hours prior to testing for transgene expression. Medium may be changed after 4-6 hours. 5. For stable cell lines: Passage cells at a 1:10 (or higher dilution) into fresh growth medium 24 hours after transfection. Add selective medium (if desired) the following day. Note: DNA and lipofectamine 2000 amounts may be varied to optimize results. *SFM-serum free medium __________________________________________________________________________________________________________________ Page 1 of 4 Safe Work Procedure Uncontrolled document when printed Date Effective: 01/01/2007 Current Version: 1.2, 15/08/2007 Procedure: Transfections with Lipofectamine™ Reagent 1. One day before transfection, plate 2-6 x 104 cells in 500 µl of growth medium (with the usual amount of serum) without antibiotics (in a 24 well plate) so that cells will be 50-80% confluent at the time of transfection. 2. For each transfection sample, prepare complexes as follows: a. Dilute 0.2-0.4 µg DNA in 25 µl of Opti-MEM® I Reduced Serum Medium (or other medium) without serum. Mix gently. b. Mix Lipofectamine™ gently before use, then dilute 0.5-5 µl in 25 µl of Opti-MEM® I Medium (or other medium) without serum. Mix gently. c. Combine the diluted DNA with diluted Lipofectamine™ (total volume = 50 µl). Mix gently and incubate for 1545 minutes at RT (solution may appear cloudy). Note: Complexes are stable for 6 hours at RT. d. For each transfection, add 0.15 ml of Opti-MEM® I Medium to the tube containing the complexes (total volume = 200 µl). Mix gently. 3. Remove the growth medium from cells and replace with 0.2 ml of growth medium without serum. Add the 0.2 ml of diluted complexes (from Step 2d) to each well. Mix gently by rocking the plate. 4. Incubate cells at 37°C in a CO2 incubator for 2-24 hours. We recommend starting with 5 hours. 5. Add 0.4 ml of growth medium containing 2X the normal concentration of serum without removing the transfection mixture. Note: If toxicity is observed after transfection, replace medium with fresh, complete medium (with normal amount of serum). 6. For transient transfection: Test for transgene activity 24-72 hours post-transfection as appropriate for your cell type and expression vector. For stable transfection: Passage cells at a 1:10 dilution into selective medium 72 hours post-transfection. Procedure: Enhancing Transfections with Lipofectamine™ Reagent 1. The day before transfection, plate cells in 24-well plates so that they are 50-80% confluent the day of transfection. At the time of plating and during transfection, avoid antibiotics. 2. Pre-complex the DNA with the Plus™ Reagent: Dilute 0.4 µg DNA into 25 µl dilution medium without serum (Dulbecco’s Modified Eagle Medium or similar medium). Mix Plus™ Reagent before use. Add 4 µl Plus™ Reagent to diluted DNA, mix again, and incubate at RT for 15 minutes. 3. Dilute 1 µl Lipofectamine™ Reagent into 25 µl dilution medium without serum in a second tube; mix. 4. Combine pre-complexed DNA (from step 2) and diluted Lipofectamine™ Reagent (from step 3); mix and incubate for 15 minutes at RT. 5. While complexes are forming, replace the medium on the cells with 0.2 ml of cell growth medium without serum. Note: It is possible to include serum in the cell growth medium at this step. 6. Add the DNA-Plus™-Lipofectamine™ Reagent complexes (from step 4) to each well of cells containing fresh medium. Mix complexes into the medium gently; incubate at 37°C at 5% CO2 for 3 hours. 7. After the 3 hours incubation, increase volume of medium to normal volume; add serum to bring the final concentration to that of normal growth medium. If necessary to maximize cell growth, replace the medium containing the complexes with fresh, complete medium after 3 h incubation or the day after transfection. 8. Assay cell extracts or stain cells in situ for reporter gene activity 24-48 hours after the start of transfection, depending on cell type and promoter activity. For stable transfection: Passage cells at a 1:10 dilution into selective medium 72 hours post-transfection. List all resources required including plant, chemicals, personal protective clothing and equipment, etc Biological Safety Cabinet Tissue culture lab coat Latex Gloves Tissue culture medium Tissue culture equipment (plates, pipettes, pipette gun, 24 well plate) 70% ethanol Lipofectamine 2000 Lipofectamine Reagent Lipofectamine Plus Reagent Selective antibiotics __________________________________________________________________________________________________________________ Page 2 of 4 Safe Work Procedure Uncontrolled document when printed Date Effective: 01/01/2007 Current Version: 1.2, 15/08/2007 List potential hazards and risk controls including specific precautions required 1- Working with human cell lines carries a potential biohazard risk. Avoid exposure to cell cultures by wearing personal protective equipment (long sleeve gown which ties up at the back and latex gloves). Clean the biological safety cabinet with 70% ethanol thoroughly after use to remove any potential biohazard within this sterile environment (see Biological Safety Cabinet SWP: STGCL.SWP.31.1 Turn on UV lamp for at least 20 minutes after use to kill any living organisms within the cabinet. 2- Selective antibiotic hazards a-Hygromycin Potential Health effects Eyes Risk of serious damage to eyes. Skin Toxic in contact with skin. Severe skin irritation. Inhalation Toxic by inhalation. Ingestion Toxic if swallowed. Correct PPE (Gloves, lab coat, face mask and glasses) and lab safety training. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice After contact with skin, wash immediately with plenty of water In case of accident or if you feel unwell seek medical advice immediately First Aid: For advice, contact a Poison Information Centre on 13 11 26 (Australia Wide) or a doctor (at once). b-Geneticin Potential Health Effects: Eye: Can cause moderate irritation, tearing and reddening, but not likely to permanently injure eye tissue. Skin: Can cause moderate skin irritation, defatting, and dermatitis. Not likely to cause permanent damage. May cause sensitization. Inhalation: Can cause moderate respiratory irritation, dizziness, weakness, fatigue, nausea and headache. May cause allergic respiratory reaction. Correct PPE (Gloves, lab coat, face mask and glasses) and lab safety training. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice First Aid: For advice, contact a Poison Information Centre on 13 11 26 (Australia Wide) or a doctor (at once). If swallowed, do not induce vomiting. List emergency shutdown instructions First Aid: For advice, contact a Poison Information Centre on 13 11 26 (Australia Wide) or a doctor (at once). List clean up and waste disposal requirements Refer to SWP for Safe Work Procedure for Waste Disposal at St George Clinical School (STGCL.SWP.2.1) List legislation, standards and codes of practice used in the development of the SWP NSW OHS Act 2000 NSW OHS Regulation 2001 AS/NZS 2243.2:2006. Safety in laboratories. Part 2: Chemical aspects AS/NZS 2243.3-2002. Safety in laboratories. Part 3: Microbiological aspects and containment facilities. __________________________________________________________________________________________________________________ Page 3 of 4 Safe Work Procedure Uncontrolled document when printed Date Effective: 01/01/2007 Current Version: 1.2, 15/08/2007 Supervisory approval, training, and review Supervisor: Prof Beng Chong (Medicine) Signature: Supervisor: Signature: Supervisor: Signature: Supervisor: Signature: Supervisor: Signature: Supervisor: Signature: Plant custodian: Prof Beng Chong Signature List competency required – qualifications, certificates, licensing, training - eg course or instruction: Induction into the lab, UNSW PC2 training Training in this SWP. __________________________________________________________________________________________________________________ Page 4 of 4 Safe Work Procedure Uncontrolled document when printed Date Effective: 01/01/2007 Current Version: 1.2, 15/08/2007