Transfection in 24
advertisement

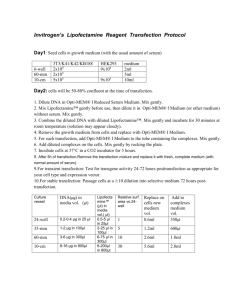
Transfection for transcriptional assays in Saos-2 cells using 24-well plates Despite I indicated the amounts of DNA and dsRNA in my Molecular Cell paper, titrate the amounts, because other factors, such as how healthy your cells are, will contribute to the effects. Too much expression will affect both luciferases. Reach consistent reproducible luciferase activity before you make conclusions. I did used pRL-CMV renilla for BRD2 and BRD8, like it is said in the paper. Saos-2 cells were splitted (1 p100 per 3-4 24-well plates) 2 days before transfection, so cells were 50-70% confluent before transfection. Regular growth media for Saos-2 is DMEM (CellGro) with 10% FBS, without antibiotics. Prepare Master Mix (MM) Lipofectamine 2000 dilution for all wells: For example, 12 l of Lipofectamine 2000 (Invitrogen) were diluted with 1 ml of OPTI-MEM (Gibco) and incubated about 5 min. For each well, dilute 1 l of Lipofectamine 2000 with 50 l of OPTI-MEM. Prepare MM DNA/siRNA dilution for all cells, then divide on your replicates: For plasmid/siRNA amounts, look in the Molecular Cell paper, for each well dilute with 50 l of OPTI-MEM. Use backbone plasmid in your “controls”, fill it up to 0.5 g-1 g with a carrier bacterial plasmid, such as pBluescript. Lipofectamine 2000/DNA/siRNA complexes: The Lipofectamine 2000 dilution was added to the DNA/siRNA dilution and the solution was incubated for 20-30 min. Change medium on plate on 0.3 ml of OPTI-MEM (do not wash cells, suck out medium and leave the plate quite “wet”, so the cells will not be cold, dry out, or stressed out otherwise during the transfer; work fast). After the incubation, the Lipofectamine 2000/DNA/siRNA was added dropwise to the cells, while gently rocking the plate. Cells were put in 5% CO2 incubator for 5 hours. After transfection, OPTI-MEM was changed on regular growth medium and cells were put back in 10% CO2 incubator for overnight recovery. Next day, cells were splitted 1:2 on the regular growth medium. Lyse 48 hr later. Use enough lysis solution to cover the whole well. Use freezing/tawing procedure to make lysates using Promega Lysis buffer for luciferase assays. Do not take cell debris into assay (centrifuge). Use 25 l per assay.