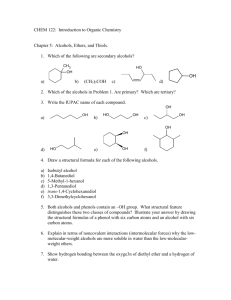
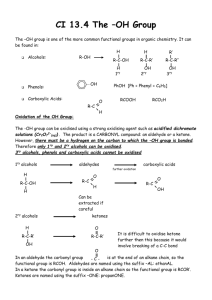
sec. 17.3 properties of alcohols/phenols: acidity & basicity
advertisement

CHAPTER 17 ALCOHOLS & PHENOLS: Generalizations: Alcohols and phenols may be thought of as organic derivatives of water (H-O-H). If one H is replaced by an alkyl group (R-), the compound is an alcohol (R-OH) and if the H of water is replaced by an aromatic group (Ar-), the compound is a phenol (Ar-OH). Although alcohols and phenols have the same functional group (a hydroxyl group), their physical properties are very different. Alcohols: Alcohols are classified as 1, 2, or 3 depending upon the number of carbon atoms bonded to the -carbon, i.e., the carbon bearing the functional group (-OH in this case). R H R C OH H 1º carbon R C OH H 2º carbon R R C OH R 3º carbon Sec. 17.1 Naming Alcohols: Common names are derived by naming the alkyl group attached to the -OH group and then, after leaving a space, name the -OH group as 'alcohol'. They are literally ‘alkyl alcohols’. IUPAC names are derived by replacing the terminal '-e' in the alkane with '-ol' giving an ‘alkanol’, written as a single word. 1. As with alkenes and alkynes, choose the longest carbon chain containing the - carbon atom, i.e., the carbon that bears the hydroxyl (-OH) group. 2. Number the chain to give -OH the lowest number. 3. List the substituents on the chain alphabetically. CH3OH CH3CH2OH CH3CH2CH2OH c methyl alcohol (carbinol) ethyl alcohol n-propyl alcohol I methanol ethanol 1-propanol OH CH3CHCH3 OH CH3CH2CH2CH2OH CH3CH2CHCH3 c isopropyl alcohol n-butyl alcohol sec-butyl alcohol I 2-propanol 1-butanol 2-butanol ALCOHOLS & PHENOLS 1 CH 3 CH 3 C OH OH CH 3CH 2CH 2CH 2CH 2OH CH 3CHCH 2CH 2CH 3 CH 3 c t-butyl alcohol n-pentyl alcohol n-amyl alcohol -------------- I 2-methyl-2-propanol 1-pentanol 2-pentanol CH 3 OH CH 3 CH 3CHCH 2CH 2OH CH 3CH 2CHCH 2CH 3 CH 3CCH 2CH 3 OH c isopentyl alcohol ------------------ t-pentyl alcohol isoamyl alcohol I 3-methyl-1-butanol t-amyl alcohol 3-pentanol 2-methyl-2-butanol OH CH 2OH OH c benzyl alcohol cyclopentyl alcohol I phenylmethanol cyclopentanol Br -------------trans-2-bromocyclohexanol Note that in cyclic alcohols, the -OH group is automatically on carbon # 1. CH 2=CH-CH 2OH OH OH OH CH 2-CH 2 CH 2-CH-CH 2 OH OH c allyl alcohol ethylene glycol I 2-propen-1-ol 1,2-ethanediol OH CH 2CH 3 CH 3 CH 3 CHCHCH 3 CH 3 OH neopentyl alcohol I 2,2-dimethyl-1-propanol ALCOHOLS & PHENOLS 1,2,3-propanetriol CH 3CCH 2OH c -----------2-ethylcyclobutanol glycerol glycerin ------------------3-phenyl-2-butanol 2 Nomenclature of Phenols: Phenols are usually named as derivatives of the parent compound, phenol. Note that phenol is both a specific compound and a class of compounds. In addition, methyl substituted phenols are called cresols while benzene diols have names based on their historical uses rather than their structures. OH OH OH c carbolic acid -naphthol -naphthol I phenol 1-naphthol 2-naphthol H3C H3C CH3 OH OH c p-cresol m-cresol I 4-methylphenol 3-methylphenol OH o-cresol 2-methylphenol HO OH OH OH c catechol resorcinol I 1,2-benzenediol 1,3-benzenediol HO OH hydroquinone 1,4-benzenediol OH H3C HO CH3 CH(CH 3)2 Br I biphenyl 2-methyl-4-(1-methylethyl)phenol 4-bromo-2-methylphenol 4-isopropyl-2-methylphenol Do problems 17.1 & 17.2 ALCOHOLS & PHENOLS 3 Sec. 17.1 Sources and Uses of Simple Alcohols: 1. Methanol (methyl hydrate, methyl alcohol, wood alcohol, carbinol) was formerly produced by pyrolysis (destructive distillation) of wood. It is commonly used as ‘gas line antifreeze’. Methanol is toxic by ingestion, causing blindness in small amounts (15 mL) and death in larger amounts. Methanol is produced commercially by catalytic reduction of CO. The catalyst is ZnO/CrO3. CO + 2 H2 400 ºC CH3OH ZnO / CrO3 2. Ethanol (ethyl alcohol, alcohol, grain alcohol) has been produced for several thousand years by fermentation of grains and sugars. Currently, most (~95%) of the ethanol produced industrially is by acid-catalyzed hydration of ethylene. H H C H + H HSO4 H - C H H2O H C H H C+ H H O H H H H C C H O+ H H H H H C C H H HSO4 - OH + H3O+ 3. Phenol occur widely in nature. Phenol itself is a general disinfectant found in coal tar; methyl salicylate is a flavoring in oil of wintergreen; urushiols are allergens in poison oak and poison ivy. OH OH OH C OCH3 O phenol methyl salicylate (methyl 2-hydroxybenzoate) OH R urushiols (R= C15 alkyl and alkenyl chains) Boiling Points: Alcohols and phenols have similar geometry to HOH. The R-O-H bond angle is approximately tetrahedral (109) and the ‘O’ atom is sp3 hybridized. Because of the presence of the hydroxyl group, alcohols (and phenols) have significantly higher boiling points than their constitutional (structural) isomers, the ethers. For example, ethyl alcohol and dimethyl ether are constitutional isomers having the same molecular weight (46). The bp of ethanol is 78 C and the bp of dimethyl ether is -24C, a difference of 102 C! The hydroxyl groups of alcohols and phenols form intermolecular hydrogen bonds. The attractive force of hydrogen bonds must be overcome when molecules in the liquid separate into the gaseous state during boiling. The bp of ethers are similar to those of alkanes of equivalent MW. For example, diethyl ether (‘Quick Start’) with MW = 44, has a bp of 35 C and n-pentane (MW = 44) is also 35 C. Similarly, phenols have higher bp’s than aromatic ethers and aromatic hydrocarbons for the same reasons as discussed above. Phenol has a mp of 43 C and toluene has a mp of -95 C. Do problem 17.3 ALCOHOLS & PHENOLS 4 H Solubility: The hydroxyl group of alcohols is the first functional group we have encountered that has a large effect on water-solubility of the organic compound. In general, alkanes, alkenes, alkynes, arenes and their halogen derivatives (alkyl halides, etc.) are insoluble in water. The presence of the -OH group makes alcohols with up to 3 carbons miscible (infinitely soluble) in water. As the carbon chain becomes longer, the solubility decreases; as it becomes more branched, the solubility increases somewhat. Hydrocarbon Solubility in Water Compound n-pentane carbon tetrachloride n-butyl bromide 1,5-hexadiene H2O Solubility (g/100 mL, 25 C) 0.05 0.08 0.06 0.10 Alcohol and Ether Solubility in Water Compound n-butyl alcohol sec-butyl alcohol isobutyl alcohol tert-butyl alcohol n-pentyl alcohol n-hexyl alcohol 1-heptanol phenol 1-octanol 1,4-butanediol 3-chloro-1-butanol dimethyl ether diethyl ether diisopropyl ether H2O Solubility (g/100 mL, 20 C) 9 12 10 miscible 2.7 0.6 0.2 6.7 0.05 miscible insoluble very soluble 7 insoluble Note that the addition of an extra hydroxyl group in diols (vs. alcohols) increases solubility but the halogen atom has the opposite effect. A compound is considered ‘soluble’ if its solubility exceeds 3 parts of compound per 100 parts of solvent, i.e., 3%. Ethers of low MW have some solubility in water because the O-atom of an ether carries a partial negative charge and is therefore a H-bond acceptor. ALCOHOLS & PHENOLS 5 SEC. 17.3 PROPERTIES OF ALCOHOLS/PHENOLS: ACIDITY & BASICITY Like water, alcohols and phenols are both weakly basic and weakly acidic, i.e., amphiprotic. As bases, they are reversibly protonated by strong acids to yield oxonium ions, ROH2+ H R .. O .. + H H + X R O .. H + X- As acids, they dissociate to a slight extent in dilute aqueous solution by donating a proton to water, generating H3O+ and an alkoxide ion, RO-, or a phenoxide ion, ArO-. R .. O .. .. H + H2O .. R .. _ O: .. + + H3O .. The acidity constants of some alcohols are listed and compared with water ... Alcohol (CH3)3COH pKa 18.00 CH3CH2OH 16.00 HOH CH3OH CF3CH2OH 15.74 15.54 (CF3)3COH p-aminophenol phenol p-nitrophenol 2,4,6-trinitrophenol 5.4 12.43 10.5 9.9 7.2 0.60 Simple alcohols, like methanol and ethanol have acidity similar to water. t-butyl alcohol is less acidic because its alkoxide anion is bulky and not easily solvated by water 2,2,2-trifluoroethanol is more acidic than ethanol because the highly electronegative F-atoms inductively withdraw electron density from the alkoxide anion thus stabilizing the anion nonafluoro-tert-butyl alcohol is quite acidic because of its 9 highly electronegative F-atoms. Because alcohols are only weakly acidic, they don't react with weak bases such as bicarbonates, ammonia, or amines and they only react to a very slight extent with metal hydroxides, e.g., NaOH. Prove this to yourself by calculating (pKeq) for several alcohols and these bases. Alcohols, like H2O, do react with alkali and alkaline earth metals (Na, Mg, etc.) and with very strong organic bases such as sodium hydride (NaH), sodium amide (NaNH2), alkyllithium reagents (RLi) such as methyl lithium (:CH3- Li+) and Grignard reagents (RMgX) such as methyl magnesium bromide (:CH3- +MgBr). Metal alkoxides themselves are strong bases that are frequently used in organic chemistry. Phenols are ca. 106 times more acidic than alcohols. They are soluble in dilute aq. NaOH soln. The phenoxide anion is resonance stabilized (negative charge is delocalized over the o- and p-positions. Electron donating groups reduce the acidity of phenols but withdrawing groups increase phenolic Do problems 17.4 and 17.5. ALCOHOLS & PHENOLS 6 Sec. 17.4, 17.5 & 17.6 Preparation of Alcohols: Alcohols can be prepared from many compounds and are likewise used to prepare many compounds... O ROH O O O RX, R-C-OH, R-C-OR, R-C-R', R-C-H, R-O-R', C=C Methods of Preparation of Alcohols: 1. Hydrolysis of Alkyl Halides: OH-, a strong base and a good nucleophile, can displace a halide from methyl and 1º alkyl halides (a nucleophilic substitution reaction) producing alcohols. The reaction does not apply to 2º and 3º alkyl halides because strong bases cause them to eliminate HX (an elimination reaction) forming alkenes. reflux CH2CH2CH2Br + NaOH (aq) CH2CH2CH2OH + NaBr CH3 SN2 CH3 CH3CHCH2I + NaOH (aq) + CH2CH2Cl CH3CHCH2OH + NaI CH2CH2OH NaOH (aq) + NaCl Reflux means boiling the system without boiling anything over, i.e., recondensing above the distillation flask with a condenser. E2 H H H Br H H C C C C H H H H H + Na+ OH- E2 H 2º alkyl halide H C C C C H H H H NaBr H + H2O Zaitsev product is major product 2. Hydration of Alkenes: Acid catalyzed hydration follows Markovnikov’s rule and is subject to rearrangement via hydride or methide shifts, e.g., 1º C+ 2º C+ 3º C+. H + H S O 4H 3C CH CH2 H 2O CH3 + CH CH3 CH3 CH CH3 O+ H S O 4H H CH3 CH CH3 OH Recall that Markovnikov alcohols can be produced by hydration of alkenes without rearrangement via oxymercuration-demercuration (1. Hg(OAc)2 in THF (aq.), 2. NaBH4) Also recall that anti Markovnikov alcohols are produced from alkenes by hydroborationoxidation (1. BH3 in THF, 2. H2O2, pH 8) Do problem 17.6 a) & b) ALCOHOLS & PHENOLS 7 3. Reduction of Aldehydes and Ketones: Sodium borohydride (NaBH4) is a safe, effective reducing agent for this reaction. Aldehydes are reduced to 1 alcohols. Ketones are reduced to 2 alcohols. Carbon-tocarbon double bonds in both these compounds are not reduced. Lithium aluminum hydride (LiAlH4) can also be used, giving higher yields, but it is explosive in water and when heated. O C H 3C H 2C H 2C 1. OH N a B H 4 , e th a n o l H 2. H 3O C H 3C H 2C H 2C + H H 1-butanol (85%) (a 1º alcohol) butanal Mechanism: .. : O: : O: R C + H H- R C + O H .. : OH H .. H H R H alkoxide C H + H2O .. H The carbonyl carbon is electrophilic (+). Hydride is a strong base (pKb = -21) and is the most powerful of nucleophiles. As hydride bonds with the carbonyl carbon, the C-to-O bond (the weakest bond) breaks (carbon can never have 5 bonds). The electronegative oxygen atom readily accepts the negative charge. In a second step, dilute aqueous acid is added. The hydronium ion protonates the alkoxide producing an alcohol and destroys any excess hydride reagent at the same time. O OH H C 1. 2. C N a B H 4 , e th a n o l H 3O + dicyclohexyl ketone 1,1-dicyclohexylmethanol (a 2º alcohol) O 2-cyclohexenone H 1. LiAlH4, ether 2. H3O+ (88%) OH 2-cyclohexen-1-ol (94%) Note that hydrides do not reduce (saturate) alkenes since alkenes are also nucleophilic. ALCOHOLS & PHENOLS 8 4. Reduction of Carboxylic Acids and Esters: These reactions require a stronger reductant than NaBH4, which reduces esters very slowly and does not reduce carboxylic acids. LiAlH4 is effective here, reducing both esters and carboxylic acids to 1 alcohols. O C H 3( C H 2) 7C H C H ( C H 2) 7C 9-octadecenoic acid (oleic acid) O CH3CH2CH CH C H 1. L i Al H 4 , e th e r C H 3( C H 2) 7C H C H ( C H 2) 7C OH + 2. H 3O H 9-octadecen-1-ol (87%) O H + H 2O 1. LiAlH4, ether OCH3 CH3CH2CH 2. methyl 2-pentenoate CH + CH2OH CH3OH + H3O 2-penten-1-ol methanol Note that 2 H's are added to a carboxylic acid from LiAlH4 and 1 H from H3O+. Note that a total of 4 H's are added when esters are reduced and two 1º alcohols are formed. H3O+ H3O+ :O: R C .. _ :O: .. O .. :H + R' _ R .. ..O C :O : OR' R R' :H H ester _ _ C + H O: R C R C _ .. O .. H + :H _ :O: 1º alcohol H2 R carboxylic acid C .. O .. : .. H2O + H O: R C 1º alcohol :O: _ :H _ _ .. AlH3 R :O : .. ..O C - AlH3 H H3O+ R :H _ H H H carboxylate C H 2 H2O :O: R H _ .. R'OH .. : O: C H _ .. : O: H R C H H H alkoxide Hydride is a strong base and so will first abstract an acidic hydrogen (proton) from the carboxylic acid. Hydride is such a strong nucleophile that it can attack even the negatively charged carboxylate anion (the carbonyl carbon in the carboxylate is only weakly electrophilic). The –- OAlH3 is a very poor leaving group (very reactive) and only leaves because H- is even more reactive. Note that aldehydes, esters and carboxylic acids all reduce to 1º alcohols with hydride. Only ketones are reduced to 2º alcohols. Do problems 17.7 & 17.8. ALCOHOLS & PHENOLS 9 5. Reduction of Carbonyls with Grignard Reagents: Grignards, RMgX, reduce carbonyl compounds to alcohols similar to LiAlH4 O O- +MgX 1. RMgX in ether C C + 2. OH H3O C R + HOMgX R Grignard reagents: When Mg metal is added to alkyl halides, aryl halides, or vinylic halides in a solvent such as ether or tetrahydrofuran (THF), Mg is inserted between the -carbon and the halide; the carbon becomes strongly electronegative (and nucleophilic). CH3-Br + Mg R-X + Mg R-MgX or in general …. CH3-MgBr where R = 1º, 2, or 3 alkyl, aryl, or vinylic where X = Cl, Br, or I Grignards are useful reducing agents. They react with formaldehyde, CH2=O, to give 1 alcohols. They react with higher aldehydes to give 2 alcohols, and with ketones and esters to give 3 alcohols. H MgBr + O 1. Mix C 2. H3O+ H H cyclohexyl magnesium bromide formaldehyde O CH3 MgBr + CH3CHCH2 H CH3 OH CH3CHCH2 C 1. Mix in ether C H 2. H3O+ H 3-methyl-1-phenyl-1-butanol (73%) (a 2º alcohol) OH O 2. H3O+ ethyl magnesium bromide cyclohexanone O CH3CH2CH2CH2 C 1-ethylcyclohexanol (89%) ( a 3º alcohol) OH 1. 2 CH3MgBr CH3CH2CH2CH2 OCH2CH3 ethyl pentanoate CH2CH3 1. Mix in ether + CH3CH2MgBr OH cyclohexylmethanol (65%) (a 1º alcohol with a longer C chain) 3-methylbutanal phenyl magnesium bromide C 2. 2 H3O+ C CH3 + CH3CH2OH + 2 MgBrOH CH3 2-methyl-2-hexanol (85%) ( a 3º alcohol) Write a complete mechanism for Grignard reduction of an ester. ALCOHOLS & PHENOLS 10 Carboxylic acids don’t give alcohols with Grignards because their acidic hydrogen reacts with the strongly basic Grignard to yield a hydrocarbon and a Mg-salt of the acid. A Grignard is not as strong a nucleophile as LiAlH4 and the carboxylate anion is not attacked by a Grignard as it is with the very powerful hydride nucleophile. Actually Grignards are sometimes used for precisely the purpose of converting an alkyl halide to an alkane via a Grignard reaction. O O CH3CH2 C + O propanoic acid CH3 MgBr CH3CH2 -+ C + O MgBr H CH4 methane Grignards also add to other compounds that have an electropositive atom, i.e., O C C N SO2R NO2 NR2 Limitations of the Grignard Reaction: Grignards cannot be prepared when reactive groups are present along with the halide, e.g., acidic H’s in carboxylic acids. Grignards are destroyed (protonated) by even weakly acidic functional groups All of the groups listed in the table below have a terminal H that is acidic enough to react with the strongly basic Grignard. ArCOOH RCOOH ArSH RSH ArOH R-OH amide -CC-H ArNH2 RNH2 4 5 7 10 10 16 17 25 ~30 35 pKa Assuming alkyl amines (pKa ~ 35) to be the weakest acid that would react with a Grignard, calculate the approximate pKb of a Grignard. Do problems 17.9, 17.10 & 17.11 Sec.17.7 + H3C Reactions of Alcohols: - .. O .. + H Alcohols, like water, are amphoteric, i.e., they can act as both acids and bases. The pKa and pKb of simple alcohols are both in the range of 16-19. In alcohols, both the -C and the hydroxyl-H are + while the hydroxyl-O is -. The lone pairs of electrons on the hydroxyl-O make it basic and nucleophilic. The + H makes it weakly acidic. a) Strong bases can abstract the weakly acidic H from alcohols producing alkoxides C O H alcohol (as acid) ALCOHOLS & PHENOLS + B strong base C O + + BH alkoxide 11 b) In the presence of strong acids, alcohols act as bases and accept protons. This is the same as what occurs when strong acids are dissolved in water, i.e., the hydroxyl-O accepts a proton from the acid and H3O+ forms. C + H O H alcohol (as base) A C H + O H + A - strong acid c) Good nucleophiles, like HS-, CN-, I-, and Br-, may attack and bond with the -carbon causing the C-O bond to break, resulting in a substitution. The hydroxyl group, however, is a poor leaving group in substitution reactions for several reasons. First, the C-OH bond is very strong (>90 kcal/mol) and is difficult to break. Second, the hydroxyl group must leave as OH- and charge separation always requires high energy input. Substitution of the OH group is much easier if the hydroxyl group is first protonated as in reaction b) above. The leaving group is then a neutral molecule, i.e., H2O. nucleophilic substitution reaction - + Nu H + O H C Nu + C H2O protonated alcohol 1. Dehydration of Alcohols to Alkenes: H OH C C H+ C dehydration C H2O + alkene alcohol 3 alcohols are dehydrated by warm (50 C) aqueous H2SO4 in THF. Elimination follows Zaitsev’s rule, producing the more highly substituted alkene. CH3 + H O H 1-methylcyclhexanol (3º alcohol) CH3 + H O H HSO4 THF 50ºC 1-methylcyclohexene E1 CH3 + H CH3 + H2O H H HSO4- 3º C+ 2 and 1 alcohols require severe conditions (conc. H2SO4 & heat!). Recall the dehydration of cyclohexanol to cyclohexene. Under these conditions, product may be charred or rearrange (if more highly substituted carbocations can form). C C C OH 20% H HSO4 50ºC C C + C t-butyl alcohol (3º alcohol) ALCOHOLS & PHENOLS C C fast E1 rxn. C + H O H C C C+ C C H 3º C+ HSO4- C + H2O + H2SO4 C 2-methylbutene 12 C C OH isopropyl alcohol (2º alcohol) C 60% H+ HSO4100ºC C C C OH ethyl alcohol (1º alcohol) + H O H C C C+ 2º C+ C HSO4- C + H2O + H2SO4 H2O + H2SO4 propene Unstable 1 C+ requires high temp. slow E1 rxn. C C H C moderate E1 rxn. 95% + H HSO4 170ºC C C + H O H C H H C+ 1º C+ C HSO4 C + ethylene A safer approach, to dehydrating 1º and 2º alcohols is to react them with phosphorus oxychloride (POCl3) in pyridine (C5H5N) - a basic amine solvent. The reaction will proceed even at 0 C. Pyridine (pKb = 5.3) is a basic solvent. The mechanism is E2 (no C+ forms and no rearrangements occur) . .. OH .. :N .. Cl + O P Cl Cl H O + P O H :N H .. : O Cl Cl P O Cl Cl cyclopentene This also works with 3 alcohols (E2) but the reagent is nasty and should be avoided if possible. Do problem 17.12 2. Conversion of Alcohols into Alkyl Halides (Sec. 17.7): This is another C-O bond breaking reaction of alcohols. HI, HBr, or HCl react readily with 3 alcohols, moderately with 2 alcohols, and poorly with 1 alcohols. For example, t-butyl alcohol reacts rapidly with conc. HCl at 25 C forming t-butyl chloride. 1 and 2 alcohols are unreactive under these conditions but 2º alcohols will react if ZnCl2 catalyst is added. These differences provide the basis of a qualitative test (Lucas test) to distinguish alcohols. Lucas reagent = ZnCl2 dissolved in conc. HCl. Formation of an alkyl chloride from an alcohol is indicated by the cloudiness that appears when the alkyl chloride separates from the aqueous solution. 3º, allyl and benzyl alcohols react in seconds (even without ZnCl2). 2º alcohols react in 1 to 5 minutes (only with ZnCl2). 1º alcohols require from 10 minutes to days to react unless heated. Note that alcohols must be soluble in Lucas reagent otherwise any alkyl halide produced will not appear as cloudiness but will simply dissolve in the organic (alcoholic) layer. Alcohols of not more than 6 carbons are soluble in the Lucas reagent. ALCOHOLS & PHENOLS 13 conc. (37%) H Cl C C C OH C C C ZnCl2 C fast SN1 rxn. C + H O H C+ C C C conc. (37%) H Cl C OH ZnCl2 moderate SN1 rxn. isopropyl alcohol (2º alcohol) C + H O H C C C OH ZnCl2 ethyl alcohol needs heat (1º alcohol) slow SN2 rxn. C C H2O C Cl- C+ Cl C C + H2O isopropyl chloride 2º C+ conc. (37%) H Cl C C C + C t-butyl chloride 3º C+ t-butyl alcohol (3º alcohol) Cl C C C C C Cl- 1º C+ + H O H C+ C not formed too unstable Cl- Cl C C + H2O ethyl chloride As in the dehydration reaction studied in the last section, 1 and 2 alcohol reactivity can be improved by reacting with a reagent which makes the -OH group a better leaving group. Phosphorus tribromide (PBr3) and thionyl chloride (SOCl2) react quickly by SN2 mechanism. Whereas reaction with HX is reversible, these reactions proceed to completion. 3 alcohols will also react quickly with PBr3 and SOCl2 but via an SN1 mechanism. Since SOCl2 and PBr3 are corrosive, we would normally choose a mineral acid (HCl, HBr or HI) to produce an alkyl halide from a 3º alcohol. R OH + O S Cl + Br ALCOHOLS & PHENOLS P Br R Cl (1º alcohol) R OH H - Cl O (1º alcohol) Br Br - R + O H + O S Cl PBr2 R R Cl + SO2 + HCl Br + HOPBr2 14 3. Reaction of Alcohols as Acids: (a qualitative lab test for alcohols) Active metals such as Na, Li, K, Ca, etc. are strong bases (as well as strong reducing agents) and will deprotonate alcohols. Alcohols act as acids in this case and form alkoxide salts. R O + H strong base weak acid CH3CH2OH R Na + Na+ + 1 + 1 /2 H2 /2 H2 alkoxide - Na - O CH3CH2O Na + sodium ethoxide H3C H3C C OH + K C H3C /2 H2 potassium isopropoxide CH3 CH3 + 2 CH3CHCH2OH Ca - + + (CH3CHCH2O )2 Ca calcium diisobutoxide isobutyl alcohol CH3 H2 CH3 CH3CCH2OH + (CH3CCH2O- )3 Al+3 Al + 3 / 2 H2 CH3 CH3 neopentyl alcohol 1 + H3C isopropyl alcohol 3 O K+ aluminum trineopentoxide Write an equation for the reaction of cyclohexanol and lithium. Name the product. Elimination Often Competes with Substitution: Strong dehydrating acids (H2SO4, H3PO4) favor elimination (dehydration) in alcohols. Because they are strong acids, they readily protonate the alcohol thereby converting a poor leaving group (OH-) into a better leaving group (HOH), however, the anions produced after protonation of the alcohol (HSO4- or H2PO4-) are very poor nucleophiles and can’t replace the leaving group, so elimination (dehydration) occurs. + H2SO4 (catalyst) CH2=CH2 + (CH3)2CH-OH + H2SO4 (catalyst) CH3CH=CH2 + H2O (elimination) (CH3)3C-OH H2SO4 (catalyst) (CH3)2C=CH2 + H2O (elimination) CH3CH2-OH + H2O (elimination) Strong non-dehydrating acids (like HI, HBr and HCl) also readily protonate an alcohol creating a better leaving group (HOH) but with the difference that the resulting Nu:-’s (like I, Br-, and Cl-), are better Nu:-’s and readily replace the leaving group which results in substitution instead of elimination. CH3CH2-OH + HBr (CH3)2CH-OH + HBr (CH3)3C-OH HBr + ALCOHOLS & PHENOLS CH3CH2-Br (CH3)2CH-Br + H2O + H2O (CH3)3C-Br + H2O (substitution) (substitution) (substitution) 15 4. Oxidation of Alcohols (Sec. 17.8): This represents the opposite of reduction of carbonyls to alcohols. (loss of H or gain of O) O C C C H C oxidation reduction O C C C (loss of O or gain of H) 1 alcohols have 2 -hydrogens and can either lose one of them to yield aldehydes or lose both of them to form carboxylic acids (depending on the oxidant strength) 2 alcohols can lose their only -hydrogen to yield ketones or under severe oxidation conditions, can be cleaved to carboxylic acids. 3 alcohols don’t have any -hydrogens and so don’t normally oxidize except under severe oxidation conditions in which case they can dehydrate to alkenes which are subsequently oxidized and cleaved to carboxylic acids. Oxidants include acidic or basic aq. KMnO4, Na2Cr2O7 in HAc, NaOCl in aq. HAc, CrO3 in aq. H2SO4 + acetone (Jones reagent), CrO3 in pyridine (Collins reagent), or PCC, i.e., pyridinium chlorochromate, (C5H5NCrO3Cl) in dichloromethane solvent. a) 1 alcohols are oxidized only to aldehydes by the anhydrous oxidants (Collins reagent or PCC) which are mild. The aqueous oxidants will oxidize 1 alcohols to carboxylic acids. mild oxidation (oxidants in non aq. solvents) PCC (in CH 2Cl 2) Collins reagent (CrO 3 in pyridine) OH R O R C H aldehyde CH2 1º alcohol O moderate or strong oxidation R C OH (oxidants in aq. solvents) Jones reagent (CrO 3 in aq. H 2SO4 & acetone) carboxylic acid KMnO 4, HNO 3, etc. b) 2 alcohols yield ketones using any of the above oxidants except the strongest (hot KMnO4, hot H2CrO4, or hot conc. HNO3) which dehydrate them to alkenes and subsequently cleave the alkenes to carbonyl compounds (See Ch. 7 on alkene cleavage) OH R CH R 2º alcohol O mild to moderate oxidation (in non aq. solvents or any cold aq. solvent) R C R PCC (in CH2Cl2) ketone Collins reagent (CrO3 in pyridine) Jones reagent (CrO3 in aq. H2SO4 & acetone) cold acidic or basic KMnO4, HNO3, etc. O H O severe oxidation R C R R C R (hot aq. KMnO4 or HNO3) ketone enol O 2 R C OH carboxylic acid c) 3 alcohols are not oxidized by any of the above oxidants except hot KMnO4 or hot conc. HNO3 which dehydrate them to alkenes and subsequently cleave the alkenes to carbonyl compounds (See Ch. 7 on alkene cleavage). 3 alcohols are easiest to dehydrate to alkenes. ALCOHOLS & PHENOLS 16 no rxn. any anhydrous or cold aq. oxidant OH R C R R 3º alcohol O severe oxidation (hot aq. KMnO 4 or HNO 3) R dehydration CH R alkene 2 R C OH carboxylic acid Do problems 17.13 & 17.14 Identifying Alcohols by Oxidation: 1. Jones Reagent: Chromic acid is a clear orange solution of Cr+6 in aq. H2SO4. When it is reduced (e.g., when it oxidizes an alcohol) the solution turns blue-green due to the formation of Cr+3 ion. 1 and 2 alcohols are reduced by Jones reagent that changes from orange to green. This is a simple qualitative test that can distinguish 3 alcohols from 1 and 2 alcohols and aldehydes. Blood Alcohol Screening: In its simplest form, a blood alcohol-screening test consists of a sealed glass tube containing a potassium dichromate reagent impregnated on silica gel. To administer the test, the ends of the tube are broken off, a mouthpiece is fitted to one end, and the other end is inserted into the neck of a plastic bag. The person being tested then blows into the mouthpiece until the plastic bag is inflated. As breath containing ethanol vapor passes through the tube, reddish orange dichromate ion is reduced to green Cr+3 ion. The concentration of ethanol in the breath is then estimated by measuring how far the green Cr+3 ion color extends along the length of the tube. When the green color extends beyond the halfway point, the person is judged as having a sufficiently high breath alcohol content to warrant more precise testing. The Breathalyzer, a more precise testing device, operates on the same principle as the simplified breath-screening test. In a Breathalyzer test, a measured volume of breath is bubbled through a solution of Jones reagent and the color change is measured spectrophotometrically. These tests measure alcohol in the breath. The legal definition of being under the influence of alcohol, however, is based on blood alcohol content. The chemical correlation between these two measurements is that air deep within the lungs is in equilibrium with blood passing through the pulmonary arteries, and an equilibrium is established between blood alcohol and breath alcohol. It has been determined by simultaneous tests that 2100 mL of breath contains the same weight of ethanol as 1.00 mL of blood. See W. C. Timmer, J. Chem. Ed., 63:897 (1986). 2. The Iodoform Reaction: The iodoform reaction, although of little synthetic value is often used to determine whether an alcohol contains a particular structural unit. The alcohol is treated with a solution of iodine and a solution of sodium hydroxide. NaOH + I2 NaOI (sodium hypoiodite) ALCOHOLS & PHENOLS 17 The product of the reaction of the suitable alcohol and the reagent is iodoform (CHI3), which is a light yellow solid with a mp of 119 C. In order for the alcohol to give a positive iodoform reaction, it must 1) be readily oxidized and 2) have methyl group attached to the carbon containing the alcohol group (the -carbon = the carbinol carbon). Of all the 1 alcohols, only ethanol, CH3-CH2-OH, meets the structural requirement. 2 alcohols are readily oxidized and those that have a methyl group attached to the carbinol carbon atom give a positive iodoform reaction ... e.g., CH3-CH(OH)-CH3 and CH3-CH2-CH(OH)-CH3 3 alcohols, although they may have two and in the case of t-butyl alcohol, three methyl groups attached to the carbinol carbon, do not give the iodoform reaction because they are not oxidized under the reaction conditions. The reaction involves oxidation, iodination, and cleavage. Consider ethanol .... O OXIDATION: + CH3 CH2 OH NaOI O IODINATION: + CH3-C-H O + CH3-C-H 3 NaOI + CI3-C-H 3 NaOH O CLEAVAGE: + H2 O NaI + CI3-C-H NaOH CHI3 O + + - Na O-C-H The reaction involving 2-butanol and NaOI proceeds as follows ... OXIDATION: CH3 CH2 CHCH3 + NaOI + NaI + CH3 CH2 CCH3 3 NaOI CH3 CH2 CCI3 3 NaOH + O O + CLEAVAGE: CH3 CH2 CCI3 + H2 O O OH IODINATION: CH3 CH2 CCH3 + NaOH CHI3 + O - Na O-C-CH2-CH3 O Question: How could one differentiate between 2-pentanol and 3-pentanol? Consider the Lucas test, the Jones reagent test and the Iodoform reaction. It is apparent from the foregoing reactions that acetaldehyde and methyl ketones will also give a positive reaction with the iodoform test. Br2 and Cl2 also react with methyl alcohols and methyl ketones to yield bromoform (CHBr3) and chloroform (CHCl3), respectively. Haloform is the general term to describe CHX3; hence the reaction is often referred to as the 'haloform' reaction. Because bromoform and chloroform are nondistinctive liquids, their formations are not useful for identification tests. ALCOHOLS & PHENOLS 18 5. Reduction of Alcohols to Alkanes: Alcohols can be reduced in 2 steps by first dehydrating to an alkene (with a strong dehydrating acid such as H2SO4 or H3PO4 and subsequently hydrogenating the alkene to an alkane. Another method is to first convert the alcohol to a tosylate ester and then displace the tosylate group with H:- using LiAlH4. H OH + Cl O O H pyridine S CH3 O O cyclopentanol LiAlH4 S CH3 O tosyl chloride, TsCl cyclopentane cyclopentyl tosylate The student should be able to write a mechanism for this reaction. 6. Protection of Alcohols (Sec. 17.9): Alcohol groups can be protected during synthesis reactions by reacting the alcohol with trimethylsilyl (TMS) chloride forming a TMS ether which is not attacked by Grignards. The TMS group can be later removed by aqueous acid or fluoride ion. Mg (CH3CH2)3 N HOCH2CH2Br + (CH3)3 Si O CH2CH2Br (CH3)3SiCl (CH3)3 Si O CH2CH2MgBr ether protect Grignard reagent O OH (CH3)3 Si OH + CH3CH 2. H3O+ OH H3O+ HO CH2CH2 CHCH3 1. (CH3)3 Si O CH2CH2 CHCH3 remove protecting group ALCOHOLS & PHENOLS 19 PHENOLS Sec. 17.10 & 11 We have already studied the synthesis of phenol by high temperature reaction of aromatic sulfonic acids with NaOH. : : O .. O .. H H .. OH .. H 1. NaOH, 300ºC 2. H3O+ H :O : H H S OH H H H H Also recall that phenols are also produced by nucleophilic aromatic substitution (NAS) of halobenzenes bearing one or more electron withdrawing groups (-NO2) ortho or para to the halogen. H H H .. Cl .. : H H NaOH NO2 OH H - : H H - H NO2 .. Cl .. : H - .. : Cl : H H NO2 H NaOH H OH + H3O OH NO2 H H .. + O .. : Na Reactions of Phenols: 1. Acidity: The hydroxyl hydrogen in phenols is acidic enough to react completely with dilute NaOH. The phenoxide ion is resonance stabilized. Draw the mechanism. Calculate pKeq, the extent of the forward reaction and the pKb of phenoxide. Draw resonance structures of phenoxide anion. O O H + + Na + + - Na OH H2O sodium phenoxide pKa = 9.9 2. Electrophilic Aromatic Substitution (EAS): The OH group is an electron donor to the aromatic ring with the result that phenols readily undergo EAS in their o- and p-positions. Pentachlorophenol, a wood preservative, is prepared via EAS by reaction of phenol & excess Cl2. Butylated hydroxytoluene (BHT), an antioxidant food preservative is prepared by Friedel-Crafts alkylation of p-methylphenol (p-cresol) with 2-methylpropene in acid catalyst. Butylated hydoxyanisole (BHA), a preservative in butter, is prepared by alkylation of p-methoxyphenol. OH Cl OH OH Cl (CH3)3 C C(CH3)3 C(CH3)3 Cl Cl Cl pentachlorophenol (PCP) CH3 BHT 2,6-bis(1,1-dimethylethyl)-4-methylphenol O CH3 BHA 2-(1,1-dimethylethyl)-4-methoxyphenol Do Problems 17.16 & 17.17. ALCOHOLS & PHENOLS 20 3. Oxidation of Phenols: (to Quinones) Phenols do not undergo oxidation like alcohols since they do not have any -carbon hydrogens. Instead, in the presence of strong oxidants such as Na2Cr2O7 [or Fremy’s salt, potassium nitrosodisulfonate, (KSO3)2NO], phenols are oxidized to quinones (2,5-cyclohexadiene-1,4-dione). Quinones are reversibly reduced to hydroquinones by reducing agents such as NaBH4 or SnCl2. O OH OH SnCl2, H2O (KSO3)2NO H2O (KSO3)2NO O OH quinone hydroquinone (1,4-benzenediol) The redox properties of quinones are vital to respiration of all living cells. Ubiquitous compounds called ubiquinones (also called coenzymes Q) react with O2 to oxidize NADH (nicotinamide adenine dinucleotide reduced) to produce NAD+ (oxidized form), water and energy. Phenol and various methylated phenols are used as antiseptics. Phenol itself is corrosive and toxic; ingesting only about 1 gram is sufficient to cause death. The largest industrial use of phenol is in the manufacture of Bakelite, a heat resistant polymer made by polymerizing phenol with formaldehyde. This same polymer is also used as an adhesive in bonding plywood. Do the following end of chapter problems: 17.21, 22, 24, 27-35, 37, 38, 41, 48 and 54. ALCOHOLS & PHENOLS 21 THIOLS Sec. 17.12 Ethers are obtained if both H’s of water are replaced by two alkyl groups (R-OR’), or by one alkyl and one aromatic group (R-O-Ar), or by two aromatic groups (Ar-O-Ar’). Thiols (R-SH) are sulfur analogs of alcohols, i.e., S replacing O. S is directly below O in Group VI of the periodic table. Many organic compounds containing O have S-analogs. Generic sulfur compounds are shown .... R R-SH thiols R-S-R Ar-SH R-S-R sulfoxide O S R-S-R O sulfone R-S-R + disulfides thiophenols thioethers O R-S-S-R trialkylsulfonium ions O O R-C-R R-S-OH R-S-OH thioketone sulfinic acid O sulfonic acid The S-analog of an alcohol (R-OH) is an ‘alkanethiol’ or a ‘thiol’ (R-SH). [‘theion’, Greek, brimstone - an older name for sulfur]. In the common nomenclature system, thiols are called ‘mercaptans’. [‘mercurinium captans’, Latin, means capturing Hg]. Mercaptans react with Hg+2 ions and the ions of other heavy metals to form precipitates. The compound CH2(SH)-CH(SH)-CH2OH, known as ‘BritishAnti-Lewisite’ (BAL) was developed as an antidote for poisoning, e.g., As, Hg, heavy metals. The ‘-SH’ group has several names 1) mercapto group 2) thiol group 3) sulfhydryl group Nomenclature: IUPAC names for thiols are named like alcohols. The suffix ‘-thiol’ is attached to the alkane name. When the -SH group is a substituent, it is identified with the prefix "mecapto" or "thio" on the parent name. They are literally named as ‘alkanethiols’. Common names of simple thiols are like those for simple alcohols except that ‘alcohol’ is replaced by ‘mercaptan’and become ‘alkyl mercaptans’. CH3SH (I) methanethiol CH3CH2CH2SH 1-propanethiol (c) methyl mercaptan n-propyl mercaptan CH2=CH-CH 2-SH BrCH2CH2CH2CH2CH2SH 5-bromo-1-pentanethiol 2-propen-1-thiol ------------------ allyl mercaptan O OH C CH3 CH 3CH 2=CHCH 2-SH ALCOHOLS & PHENOLS CH3CHCH2CH2SH HS-CH 2CH 2-OH SH SH 22 (I) 2-butene-1-thiol 3-methyl-1-butanethiol 2-mercaptoethanol 2-thioethanol (c) -------------- isopentyl mercaptan ------------ cyclohexanethiol 3-mercaptobenzoic acid cyclohexyl mercaptan m-mercaptobenzoic ALCOHOLS & PHENOLS acid 23 Odor Properties: The most distinctive characteristic of thiols is their rank odor. H2S has the odor of rotten eggs 3-methyl-1-butanethiol and 2-butene-1-thiol are the main ingredients in skunk odor. 1-propanethiol is the odor of freshly chopped onions allyl mercaptan is a major contributor to the odor and flavor of garlic methanethiol is added to natural gas (odorless methane) to give it an odor for detecting leaks. The human nose is very sensitive to these compounds and can detect their presence at ~ 0.02 ppb in air. Higher MW thiols, being less volatile, are less odiferous. Boiling Points: S-atoms are less EN than O-atoms and thus form weaker H-bonds than alcohols. Thus low MW thiols have lower bp’s than corresponding alcohols ... CH3CH2OH (78 C) CH3OH (65 C) CH3CH2SH (37 C) CH3SH (6 C) Acidity: The bond dissociation energy of the S-H bond of thiols (~ 80 kcal/mol) is significantly less than for O-H bonds (~100 kcal/mol). In addition, S’s large size makes it more polarizable and hence better able to stabilize a negative charge than oxygen. As a result, thiols are more readily deprotonated by bases (i.e., more acidic) than alcohols ... .. ..S R + H Na+ OH- R .. _ ..S : Na+ + H2O compound pKa compound pKa H2O 15.7 H2S 7.0 CH3OH 15.4 CH3SH ~8 CH3CH2OH 16.0 CH3CH2SH 8.5 Thiols are sufficiently strong acids that when dissolved in aq. NaOH, they are converted completely to alkylsulfide salts (also called alkanethiolates). CH3CH2 .. ..S H + Na+ OH- CH3CH2 .. _ ..S : Na+ + H2O sodium ethyl sulfide sodium ethylthiolate Write the equations for the reaction of ethanethiol with NaOH (aq) and with NaHCO3, calculate pKeq and the extent of these reactions. ALCOHOLS & PHENOLS 24 Solubility: Like H2S and its salts (Na+SH-), thiols form water-insoluble salts with most heavy metals. 2 RSH + Hg+2 Hg(SR)2 + 2 H+ 2 RSH + Pb+2 Pb(SR)2 + 2 H+ (yellow precipitate) The yellow precipitate is a positive test for a thiol. Preparation of Thiols: Since S is large and polarizable, alkyl sulfide anions (RS-) and hydrosulfide anions (HS-) are good nucleophiles. These anions readily replace halogens in alkyl halides via SN2 reactions. H2S + NaOH Na+HS- + HOH CH3-Br + Na+HS- CH3-SH + NaBr The thiol product is also acidic and reacts with more base to produce sodium methyl sulfide which is a good nucleophile and can itself substitute for Br in another molecule of alkyl halide producing a dialkyl sulfide. CH3-SH + NaOH Na+CH3S- + HOH Na+CH3S- + CH3-Br CH3-S-CH3 + NaBr To avoid the 2nd reaction, a large excess of H2S may be used. Oxidation of Thiols: When alcohols are treated with oxidizing agents, oxidation takes place at the weaker C-H bond (~100+ kcal/mol) rather than at the stronger O-H bond (~ 105+ kcal/mol). H O [O] CH3 C OH CH3 C OH H In thiols, the weaker S-H bond (~ 80 kcal/mol) is broken during oxidation. Mild oxidizing agents such as I2, Br2, and potassium ferricyanide [K3Fe(CN)6], break the S-H bond and cause the formation of a disulfide containing an S-S linkage (an oxidative coupling reaction). I2 CH3CH2 S 2 CH3CH2 SH Zn, HCl S CH2CH3 + 2 HI diethyl disulfide The reaction can be reversed with reducing agents such as Zn in HCl. Molecular oxygen can also cause oxidative coupling and thiols must be protected from exposure to air to avoid this reaction. ALCOHOLS & PHENOLS 25 Stronger oxidants such as KMnO4 and H2O2 yield more highly oxidized products such as sulfonic acids. A sequence of oxidation products is shown... [O] RSH + R SOH sulfinic acid + 2 H2O thiol O [O] R SOH + O + 4 e- O R SOH H2O 4 H+ + 2 H+ + 2 e- O sulfonic acid Note that as a 3rd-row element with d-orbitals, S’s valence shell can be expanded to accommodate more electrons than allowed by the octet rule. Some S-compounds accommodate 10 or even 12 valence electrons, e.g., SF6. A sulfide (R-S-R) can be oxidized to a sulfoxide or a sulfone depending upon the reaction conditions. H+, 25 ºC O CH3 S CH3 CH3 S CH3 + dimethyl sulfoxide (DMSO) H2O2 O dimethyl sulfide + H , 100 ºC CH3 S CH3 dimethyl sulfone O DMSO is a powerful solvent for both inorganic and organic ions. It has a high dielectric constant (49D), i.e., it is highly polar, yet it does not H-bond. (Why?) DMSO solvates cations but not anions, leaving anions as bare, reactive nucleophiles-excellent for substitution reactions. DMSO readily penetrates the skin and has been used to promote dermal absorption of drugs; but it can also cause absorption of dirt and poisons. Do problems 17.18 & 17.19 ALCOHOLS & PHENOLS 26 Homework Problems from McMurry Organic Chemistry In the following tables are listed; problems ‘you should be able to solve’ and ‘problems you are not responsible for’. If you haven’t time to do all of the former questions, be sure to do a representative number, i.e., a couple of each kind until you are satisfied that you can solve these. It is recommended that you review the notes (text and class notes) and then do the problems. Until you have attempted the problems you really don’t know if you know what you should know! Homework Problems from Ch. 17 (Alcohols & Thiols) Problems you should be able to solve 1-5 7-10 11 a), c) 12-14 18-19 20-23 24 a) - e) 25 27-28 30-31 33 a) - c) 34 38 39 b) - d) 52 Problems you are not responsible for 6 11 b) 15-17 24 f) 26 29 32 33 d) 35-37 39 a) 40 - 51 and 53 - 55 The horizontal line in the table separates the in-chapter problems from the end-of-chapter problems. The answers to the in-chapter problems are the back of the text. The solutions to all end-of chapter problems are found in the solution manual for the McMurry textbook. Several copies are on sale in the bookstore and one copy is kept on reserve in the college library. ALCOHOLS & PHENOLS 27