nmr[1]molecule image..
advertisement
![nmr[1]molecule image..](http://s3.studylib.net/store/data/007850632_2-ff5f9c628b70c13d29c7f8ef83a564a9-768x994.png)
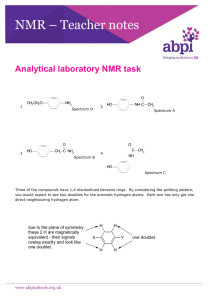
This is how the mouse over needs to be: The numbers adjacent to the H’s in the first picture correspond to the placement of the vertical peaks on the chart (the second picture). These same values are also written out in the table (3rd picture). We want a box to appear over region in which the corresponding peak lies in the 2nd picture AND we want the corresponding value in the table to be highlighted each time the mouse is passed over the H in the first picture representing that peak. There is one such example of how one of these mouseovers should look on the following page. There should be one such mouseover for each H in the first picture. There should be 18 mouseovers total. In addition, the peaks are listed in the table according to the values in the first picture from top to bottom. So although there are duplicate values in the first picture next to different H’s, the peak highlighted in the table should be different. For example, there are two H’s labeled 1.9, for the H near the top of picture 1, highlight the value in the top of the table. Similarly, for the H in the middle of picture 1, make bold the value in the middle of the table. ChemNMR 1H Estimation ChemNMR 1H Estimation 1.9 1.41 H 1.3 H 1.41 1.4 1.1 H H 1.2 1.9 H H H H 1.41 H H H O3.6 1.3 H 1.3 H 1.9 1.4 1.1 H H O H 1.1 2.4 O H 5.79 1.9 1.41 1.1 2.4 H 1.6 O 4.88 H 1.2 1.9 H H O H 1.9 O H H H O3.6 1.3 H 5.79 H H H 1.6 O 4.88 H 2.0 2.0 H H 4.95 4.95 Estimation quality: blue = good, magenta = medium, red = rough Estimation quality: blue = good, magenta = medium, red = rough 4.95 Estimation quality: blue = good, magenta = medium, red = rough 6 5 4 3 PPM 2 1 0 Protocol of the H-1 NMR Prediction: Node Shift Base + Inc. CH3 1.41 CH3 1.41 0.86 0.50 0.05 0.86 0.50 0.05 1.60 0.00 ? 0.18 -0.02 -0.03 0.33 -0.01 0.32 -0.01 H 2.4 H 4.95 H 4.88 H 5.79 H 2.0 H 1.9 Comment (ppm rel. to TMS) methyl 2 beta -O-C 1 beta -C methyl 2 beta -O-C 1 beta -C 1-cyclohexane 1 -OC(=O)C ax 1 unknown substituent(s) 1 -O eq 1 -O ax 1 -C eq 1 -C ax 1 beta -C from methine 1 beta -O-C from methine 1 beta -C from methine -> 1 increment(s) not found 5.25H-NMR 1-ethylene Multiplicity Integration -0.30 1 -1:CCCCCC-1 cis 5.25 1-ethylene -0.37 1.41 1 -1:CCCCCC-1 singlet trans 3 5.25 1-ethylene 0.54 1 -1:CCCCCC-1 gem 1.60 1-cyclohexane doublet of doublet 1.90 1 -OC(=O)C ax 2 0.70 of doublet of ? 1 unknown substituent(s) -0.27 1 -O ax 0.00 1 -C eq -0.02 1 -C ax -> 1 increment(s) not found 1.12 1-cyclohexane Reasoning This group of hydrogens is more than 3 bond neighbors with any hydrogen. In addition the 3 hydrogens do not split eachother because they are homotopic and chemically identical. There are four distinct hydrogens splitting this hydrogen (including the hydrogen on the same carbon because the two doublets 1.41 singlet 3 2.40 doublet of doublet of doublets 1 1.30 doublet of doublet of doublets 1 1.10 doublet of doublet of doublet of doublets 1 1.90 doublet of doublet of doublet of doublets 1 1.60 doublet of doublet of doublet of doublets 1 1.30 doublet of doublet of doublet of doublets 1 3.60 doublet of doublets 1 4.88 4.95 5.79 1.90 doublet of doublets doublet of doublets doublet of doublets doublet of doublet of doublets 1 1 1 1 H’s are diastereotopic) This group of hydrogens is more than 3 bond neighbors with any hydrogen. In addition the 3 hydrogens do not split eachother because they are homotopic and chemically identical. This hydrogen is 3 bond neighbors with 2 diasterotpic hydrogens on the adjacent carbon and 1 hydrogen on the other adjacent carbon. As it is being split by 3 chemically distinct hydrogens it is a ddd. Theis hydrogen is 3 bond neighbors with 3 chemically distinct hydrogens at 2.4, 1.1 and 1.9 ppm the last two of which are diastereotopic resulting in a ddd. This hydrogen split by 3 chemically distinct hydrogens (two of which are diastereotopic) to which it is a 3 bond neighbors. It is also part of a diastereotopic pair and is therefore split by the hydrogen with which it shares a carbon making it a dddd. This hydrogen split by 3 chemically distinct hydrogens (two of which are diastereotopic) to which it is a 3 bond neighbors. It is also part of a diastereotopic pair and is therefore split by the hydrogen with which it shares a carbon making it a dddd. This hydrogen split by 3 chemically distinct hydrogens (two of which are diastereotopic) to which it is a 3 bond neighbors. It is also part of a diastereotopic pair and is therefore split by the hydrogen with which it shares a carbon making it a dddd. This hydrogen split by 3 chemically distinct hydrogens (two of which are diastereotopic) to which it is a 3 bond neighbors. It is also part of a diastereotopic pair and is therefore split by the hydrogen with which it shares a carbon making it a dddd. This hydrogen is split by 2 diastereotopic hydrogens on an adjacent carbon. These two hydrogens are the only ones within 3 bonds. this hydrogen is split by the hydrogen on the same carbon at 4.95 and its 3 bond neighbor, the hydrogen at 5.79 this hydrogen is split by the hydrogen on the same carbon at 4.88 and its three bond neighbor, the hydrogen at 5.79 this hydrogen is split by its 3 bond neighbors, 2 diastereotopic hydrogens at 4.88 and 4.95. this hydrogen is split by a diastereotopic pair and is itself part of a diastereotopic pair. Coupling with 3 other chemically distinct 2.00 1.20 1.10 1.40 doublet of doublet of doublets doublet of doublet of doublet of doublet of doublets doublet of doublet of doublet of doublet of doublets doublet of doublet of doublet of doublets 2 1 1 1 hydrogens makes it a ddd. this hydrogen is split by a diastereotopic pair and is itself part of a diastereotopic pair. Coupling with 3 other chemically distinct hydrogens makes it a ddd. this hydrogen is split by 2 diastereotopic pairs (4 chemically distinct hydrogens) and is itself part of a diastereotopic pair, thus making it a ddddd as it is split by 5 other distinct hydrogens. this hydrogen is split by 2 diastereotopic pairs (4 chemically distinct hydrogens) and is itself part of a diastereotopic pair, thus making it a ddddd as it is split by 5 other distinct hydrogens. This hydrogen split by 3 chemically distinct hydrogens (two of which are diastereotopic) to which it is a 3 bond neighbors. It is also part of a diastereotopic pair and is therefore split by the hydrogen with which it shares a carbon making it a dddd. Estimation quality: blue = good, magenta = medium, red = rough 6 5 4 3 PPM 2 Protocol of the H-1 NMR Prediction: H-NMR 1.41 Node 1.90 1.41 CH3 2.40 1.30 1.10 CH3 1.90 1.60 H Shift Base + Inc. 1.41 0.86 0.50 0.05 0.86 0.50 0.05 1.60 0.00 ? 1.41 2.4 Comment (ppm rel. to TMS) methyl 2 beta -O-C 1 beta -C methyl 2 beta -O-C 1 beta -C 1-cyclohexane 1 -OC(=O)C ax 1 unknown substituent(s) 1 0 1.30 3.60 4.88 4.95 5.79 1.90 2.00 1.20 1.10 1.40 ChemNMR 1H Estimation 1.9 1.41 H 1.1 2.4 1.3 H 1.41 1.4 1.1 H H 1.2 1.9 H H O H 1.9 O H H H 3.6 O 1.3 H 5.79 H H H 1.6 O 4.88 H 2.0 H 4.95 Estimation quality: blue = good, magenta = medium, red = rough