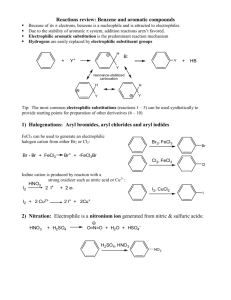
Chapter 13: NMR spectroscopy
advertisement

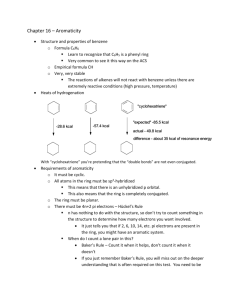
Chapter 14: Aromatic Compounds Learning Objectives: 1. Recognize and distinguish between aromatic and antiaromatic compounds by their structures. 2. Know the properties of aromatic and antiaromatic compounds, and the chemical consequences of aromaticity. 3. Recognize and be able to write the mechanism of electrophilic aromatic substitution 4. Be able to outline the completed electrophilic aromatic substitution reactions of the following types: halogenation, nitration, sulfonation, and Friedel-Crafts acylation & alkylation Sections: 14.1 14.2 14.3 14.4 14.5 14.6 14.7 14.8 14.9 14.10 14.11 14.12 14.13 14.14 14.15 14.16 14.17 14.18 14.19 Armoatic Compounds Are Unusually Stable The Two Criteria for Aromaticity* Applying the Criteria for Aromaticity Aromatic Heterocyclic Compounds Some Chemical Consequences of Aromaticity* Antiaromaticity A Molecular Orbital Description of Aromaticity and Antiaromaticity Nomenclature of Monosubstituted Benzenes How Benzene Reacts* The General Mechanism for Electrophilic Aromatic Substitution Reactions* Halogenation of Benzene* Nitration of Benzene* Sulfonation of Benzene* Friedel-Crafts Acylation of Benzene* Friedel-Crafts Alkylation of Benzene* Alkylation of Benzene by Acylation-Reduction* Using Coupling Reaction to Alkylate Benzene# It Is Important to Have More Than One Way to Carry Out a Reaction How Some Substituents Can Be Chemically Changed* * Sections that will be focused # Sections that will be skipped Recommended additional problems 14.30 – 14.49 1 Class Note 14.1 Armoatic Compounds Are Unusually Stable H2 Ni H = -28.6 kcal/mol + 3H2 cyclohexatriene (hypothetical) 36 kcal/mol + 3H2 -85.8 kcal/mol (-28.6x3) Energy + H2 -49.8 kcal/mol -28.6 kcal/mol 2 14.2 The Two Criteria for Aromaticity* and 14.6 Antiaromaticity A. Cyclic molecule B. Every atom has p orbital C. Planar molecule D. Comply with 4n+2 rule (as compared with 4n rule) E. Consequence of aromaticity 3 14.3 Applying the Criteria for Aromaticity and 14.4 Compounds 4 Aromatic Heterocyclic H N H N O S N O S H N N N N H N CH3 OH H N N N CH2 CH2 O OH O OH H N N 5 14.5 Some Chemical Consequences of Aromaticity* A. Acidity of protons H H H H B. Aromaticity on leaving group Br Br 6 C. Aromaticity on dipole moment (polarity) O O 7 14.7 A Molecular Orbital Description of Aromaticity and Antiaromaticity 8 14.8 Nomenclature of Monosubstituted Benzenes Cl NO2 Br CH3 SO3H NH2 OH OCH3 CH3 CHO CO2H As substituent: CH2 9 CHCH3 14.9 How Benzene Reacts* and 14.10 Aromatic Substitution Reactions* The General Mechanism for Electrophilic Addition vs. Substitution Electrophilic Aromatic Substitution vs. Nucleophilic Aromatic Substitution 10 A. General Mechanism of Electrophilic Aromatic Substitution B. Reaction coordinate diagram 11 14.11 Halogenation of Benzene* 14.12 Nitration of Benzene* 12 14.13 Sulfonation of Benzene* 14.14 Friedel-Crafts Acylation of Benzene* 13 14.15 Friedel-Crafts Alkylation of Benzene* Result from carbocation migration 14 14.16 Alkylation of Benzene by Acylation-Reduction* and 14.18 Have More Than One Way to Carry Out a Reaction A. Clemmensen reduction B. Wolff-Kishner reduction 15 It Is Important to 14.19 How Some Substituents Can Be Chemically Changed* A. Reactions of Alkyl Substituents tert-BuO Br NBS OH peroxide or h CN 16 H2, Pt N O H2, Pt H2, Pt B. Oxidations of Alkyl Substituents 1. KMnO4, heat (reflux) 2. H+ Na2Cr2O7, H+, heat 17 Na2Cr2O7, H+, heat Na2Cr2O7, H+, heat Na2Cr2O7, H+, heat OH MnO2 (no heating needed) OH Na2Cr2O7, H+, heat 18 C. Reduction of Nitro Group NO2 H2, Pt NO2 Sn, HCl NO2 Fe, HCl 19