Chapter 16 - People.vcu.edu
advertisement
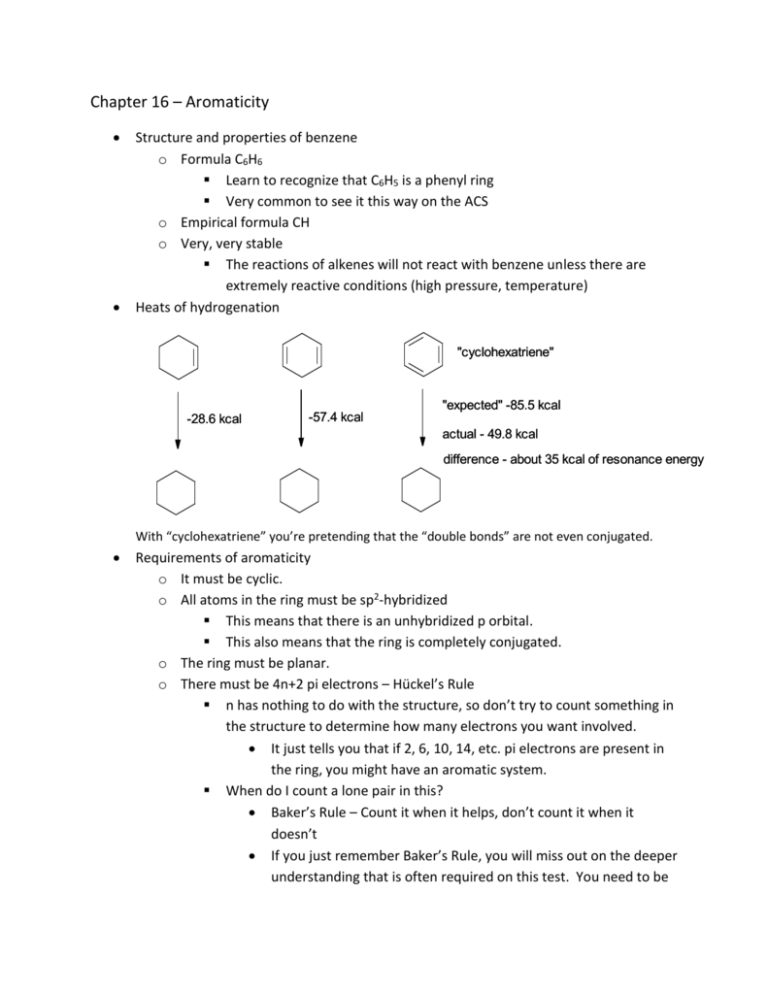
Chapter 16 – Aromaticity Structure and properties of benzene o Formula C6H6 Learn to recognize that C6H5 is a phenyl ring Very common to see it this way on the ACS o Empirical formula CH o Very, very stable The reactions of alkenes will not react with benzene unless there are extremely reactive conditions (high pressure, temperature) Heats of hydrogenation "cyclohexatriene" "expected" -85.5 kcal -28.6 kcal -57.4 kcal actual - 49.8 kcal difference - about 35 kcal of resonance energy With “cyclohexatriene” you’re pretending that the “double bonds” are not even conjugated. Requirements of aromaticity o It must be cyclic. o All atoms in the ring must be sp2-hybridized This means that there is an unhybridized p orbital. This also means that the ring is completely conjugated. o The ring must be planar. o There must be 4n+2 pi electrons – Hückel’s Rule n has nothing to do with the structure, so don’t try to count something in the structure to determine how many electrons you want involved. It just tells you that if 2, 6, 10, 14, etc. pi electrons are present in the ring, you might have an aromatic system. When do I count a lone pair in this? Baker’s Rule – Count it when it helps, don’t count it when it doesn’t If you just remember Baker’s Rule, you will miss out on the deeper understanding that is often required on this test. You need to be looking at the hybridization of the given atom and understand where the lone pair is. For instance, if a nitrogen is already involved in a double bond, then you know that the p orbital is already occupied. This means that the lone pair is in an sp2 orbital and cannot be involved in the pi system Aromatic ions o Carbocations have an empty p orbital not aromatic aromatic Why is 7-bromocyclohepta-1,3,5-triene water-soluble? aromatic not aromatic o Carbanions can become sp2-hybridized if that makes it aromatic. This means the lone pair is in the p-orbital, so you count it. This is why cyclopentadiene is a particularly acidic hydrocarbon. The pKa is around 15! NaOH not aromatic aromatic o Boron with three bonds has an empty p-orbital. If any part of a molecule is aromatic, it is aromatic!!! o This means that if you are circling the aromatic structures from a group of molecules, a huge molecule with one phenyl group is indeed aromatic. Circle it! Aromatic compounds you need to know (I’m not sure what flash cards are used for, but I think this might be one of the times when they are useful) o Pyrrole The lone pair is in a p orbital, so it is included in the pi system. o Pyridine The lone pair is in an sp2 orbital, thus not involved in the pi system o Pyrimidine Both lone pairs are in sp2 orbitals, thus not involved in the pi system o Purine One lone pair is involved in the aromaticity. o Furan One of the lone pairs of oxygen is sp2 and the other is in a p-orbital. o Naphthalene o Anthracene o Benzene Derivatives Phenol Toluene Aniline NH2 Anisole Styrene Benzoic acid Benzaldehyde Benzene sulfonic acid Nomenclature of Benzene Derivatives o If a substituted benzene has no functional group that gives it one of the common names above, then name it as a substituted benzene. bromobenzene isopropyl benzene ethoxybenzene o Ortho, meta, and para positions When you have two and only two substituents, you can use the terms ortho, meta, and para These terms tell you where two pieces are relative to one another ortho meta para o With three or more substituents, you need to go back to numbering just like you would with other naming. o Naming benzene derivatives where part of it has a common name If one of the substituents gives you a common name, then that is the “parent.” NH2 meta-chloroaniline When there are other substituents, count from the carbon whose substituent gives the parent its name. If you have two competing parents, then most likely you have a new common name, for which you are not responsible. Ex. You wouldn’t have to name this. OH H2N Polarity of aniline (more fun with resonance) There are two more resonance forms with the negative charge at the two ortho positions as well. Review of acidity of phenols o Phenol by itself has a pKa of 10. o Every nitro group you add to an ortho or para position decreases the pKa by about 3 log units. Allotropes of carbon o Diamond – all carbons sp3-hybridized Each diamond is essentially one huge molecule. o Graphite – lattice of fused aromatic rings The layers are not covalently bonded, so they slide off each other easily. o Bucky balls – balls of alternating six- and five-membered rings Named after Buckminster Fuller, the architect who was a big proponent of geodesic domes in housing. o Carbon nanotubes – very similar in structure to the Bucky ball Oh, the awesomeness of carbon nanotubes. Mmmmm. Possible solution to the space elevator?











