(InNb)O 6
advertisement

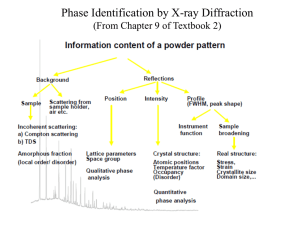
A PHASE ANALYSIS, TEM AND POWDER DIFFRACTION STUDY OF THE Ca2(InNb)O6 – Ca3(CaNb2)O9 SOLID SOLUTION V.Ting, Y. Liu, R. L Withers and L. Norén Research School of Chemistry, Australian National University, Canberra, A.C.T, Australia Recent investigation [1] of certain A2(InNb)O6 (A = Ca2+, Sr2+, Ba2+) 1:1 perovskites showed that these materials, being photocatalytic, can potentially be used as visible light-driven semiconductor photocatalysts for the evolution of H2 from water for hydrogen fuel cells. While attempting to synthesise the Ca2(InNb)O6 1:1 material, it was common to also get a small amount of an In2O3 second phase. Thus it was discovered that an extensive solid solution field exists for materials having compositions which lie on the line between Ca2(InNb)O6 and Ca3(CaNb2)O9 in the CaO-InO3/2-NbO5/2 ternary phase diagram and having chemical composition Ca6(In3-3xCa2xNbx)Nb6O18. This solid solution field is thought to stop short of the true Ca2(InNb)O6 composition. Samples were produced via solid-state reaction and analysed using the following complementary techniques: room temperature Transmission Electron Microscopy, Electron Probe Micro Analysis (EPMA), XRD and neutron powder diffraction (at Lucas Heights). The XRD patterns indicated that the materials with intermediate compositions formed single-phase compounds, for which it seems that an underlying monoclinic (a = ap+bp, b = –ap+bp, c = 2cp w.r.t cubic perovskite, subscript p) structure is maintained. Refinement of neutron powder diffraction patterns on two of these compounds shows that the cooperative b-b-c+ BO6 octahedral tilt scheme (as described by Glazer [2] and reported as being the most commonly reported tilt scheme in perovskites) is also present in these structures, and the degree of these rotations is similar to those found in pure Ca2(InNb)O6. In the TEM, for the compositions approaching the Ca2(InNb)O6 end member, the G 1/2<111>p* satellite reflections seen were believed to be the result of a rock salt-type ordering scheme for the B-site cations, as well as a further distortion in the form of rotations of the rigid octahedra. The Rietveld refinement of the neutron powder diffraction pattern confirmed this model. The electron diffraction patterns from the compositions approaching the Ca3(CaNb2)O9 end member, on the other hand, were identical to those obtained from the “high temperature” Ca3(CaNb2)O9 phase reported by Levin et al.[3] and had clear G 1/4<111>p* satellites, representative of layered 1:2 type ordering. The fact that such a large solid solution field can exist is testimony to the well-known flexibility of the perovskite structure. If the compositions of these materials are able to be changed continuously there is a possibility that the physical properties of this material might also continuously change, thus presenting the possibility for tuneable functional materials, where compositions may be varied to control the properties. References: 1. 2. 3. J. Yin, Z. Zou and J. Ye, J. Phys. Chem.. B, 107, 61-65 (2003) A.M. Glazer, Acta Crystallogr. B. 28, 3384-3392 (1972) I. Levin, L.A. Bendersky,J.P. Cline, R.S. Roth, T.A. Vanderah, J. Solid State Chem. 150, 43-61 (2000)