Crystallography 13
advertisement
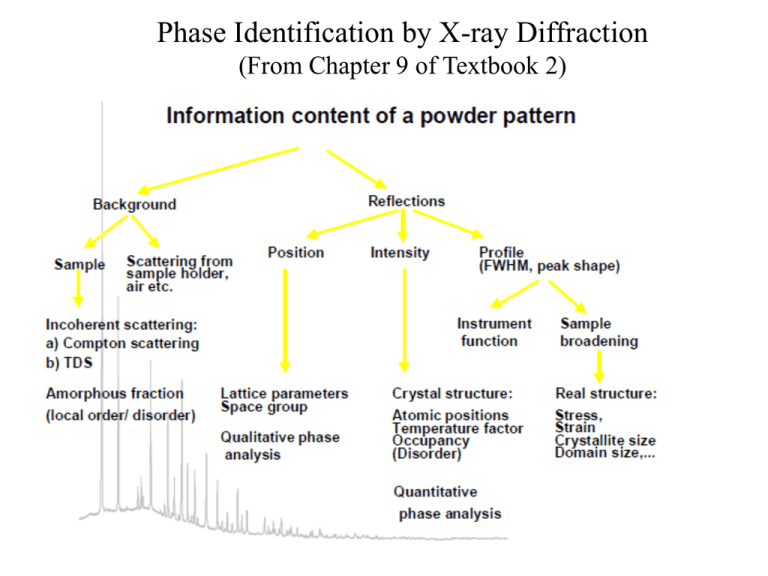
Phase Identification by X-ray Diffraction (From Chapter 9 of Textbook 2) Powder Diffraction Methods • Qualitative Analysis – Phase Identification • Quantitative Analysis – Lattice Parameter Determination – Phase Fraction Analysis • Structure Refinement – Rietveld Methods • Structure Solution – Reciprocal Space Methods – Real Space Methods • Peak Shape Analysis – Crystallite Size Distribution – Microstrain Analysis – Extended Defect Concentration 1930’s Hanawalt, Rinn and Frevel (Dow Chemical): diffraction data on about 1000 compounds JCPDS, ICDD: Joint Committee on Powder Diffraction Standards; 1978 was renamed International Center for Diffraction Data. Hanawalt Method: (Grouping scheme) values of the three strongest lines (d1, d2, d3) and intensities (I/I1) File number three strongest lines lowest-angle line Chemical formula and name of substance Special symbol data on diffraction method used crystallographic data optical and other data data on specimen diffraction pattern Special symbols give extra information: *: well-characterized chemistry, quantitative measure of intensity, high-quality d-spacing data (3 to 4 significant digits, no serious systematic errors) i: reasonable range and even spread of intensity, “sensible” completeness of the pattern, good d-spacing data (3 significant digits) o: low precision data, possible multi-phase mixture, possible poor chemical characterization c: powder pattern calculated from structural parameters Procedure (1) Locate proper d1 group (2) Find the closest match to d2 (±0.01 Å) (3) Follow by matching d3 (4) Compare relative intensity (5) Good agreement in search manual locate the proper PDF card compare the d and I/I1values of all the peaks Examples: unknown pattern from measurement: strongest lines in the powder pattern: d1 = 2.82; d2 = 1.99; d3 = 1.63 Portion of the ICDD Hanawalt search manual: d1 = 2.82; d2 = 1.99; d3 = 1.63 Matched, turn to card number 5-628 Very weak K (220) plane higher Intensity? Discrepancies!! 2×2.18×sin = 1.54 = 20.68o 2×d×sin 20.68o = 1.392 d = 1.97 Not listed Absorption effect Identification of Phases in Mixtures Examples: pattern of unknown d: 3.01 2.47 2.13 2.09 1.80 1.50 1.29 1.28 I/I1: 5 72 28 100 52 20 9 18 d: 1.22 1.08 1.04 0.98 0.91 0.83 0.81 I/I1: 4 20 3 5 4 8 10 No substance matching (d1:2.09; d2:2.47; d3: 1.80) all together probably a mixture Assume: d1 and d2 not the same phase. d1 and d3 the same phase find Cu Check the Pattern of Cu: d: 2.088 1.808 1.278 1.090 1.044 0.904 0.830 0.808 I/I1: 100 46 20 17 5 3 9 8 Remainder of pattern of unknown: d: 3.01 2.47 2.13 1.50 1.29 1.28 0.98 I/I1: 5 72 28 20 9 4 5 I/I1: 7 100 39 28 13 6 7 Normalized Following the steps of searching again Cu2O Overlapped diffraction lines carefully subtract the intensity from the already identified phases to help further identification of other phases. Example Computer searching of the PDF: Computerization has dramatically improved the efficiency of searching the JCPDS database Cards are no longer printed –data are on CD-ROM Numerous third-party vendors have software for searching the PDF database Computerized “cards” can contain much more crystallographic information Database is still expanding … New approach – whole pattern fitting Special symbol Searching of the PDF requires high-quality data Accurate line positions are a must! Calibration of camera and diffractometer with standards Careful measurement of line intensities Elimination of artifacts (e.g. preferred orientation) Solid solutions and strains shift peak positions “Garbage in, garbage out” Errors in database EVA software TOPAS software











