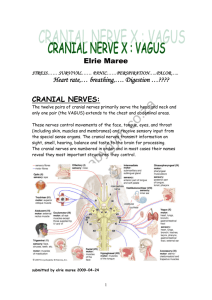
REGULATION OF PULMONARY VENTILATION
advertisement

REGULATION OF PULMONARY VENTILATION BACKGROUND In this experiment you will examine some of the factors controlling respiration. You will determine how minute volume, tidal volume, peak flow velocity and respiratory rate are modified by: inspired gas composition inspiratory resistance expiratory resistance dead space volume the activity of vagal afferents plasma pH The tracheal cannula will be fitted with a flowmeter. The flowmeter works on the principle that if the drop in pressure as the air stream moves across a measured resistance is known, then the flow rate is known from the universal relationship between force, resistance and flow. The instrument will have been calibrated beforehand to convert airflow to volume. Before connecting the transducer to the animal, you must carefully zero the amplifier so that the instrument reads zero when there is no flow. When the transducer is connected to the animal, positive flow rate values correspond to inspiration and negative ones to expiration. For each experimental condition (except the final one, lactic acidosis, where multiple recordings will probably be useful) you should take a 15 second recording when the effect of the treatment is maximal and the effect has become stable. During the administration of the series of gas mixtures, the stage of anesthesia of the animal will probably change. The animal's responsiveness to the test conditions will increase as the anesthesia wears off, and decrease if you give him additional anesthetic. Administer anesthetic judiciously, be sure the animal has returned to baseline after each test gas, and collect baseline record between each gas mixture. The interim baseline records will be the basis for determining the magnitude of the effect of each test gas. PROCEDURE 1. Anesthetize the animal with urethane. 2. A length of large-diameter PE tubing will be used as the tracheal cannula. Prepare the cannula by making a mark on it with a lab marker about 1 inch from the end that goes into the animal. This will allow you to avoid inserting the cannula too far into the trachea, and to detect slippage of the cannula after insertion. 3. Cannulate the trachea. 4. Cannulate the jugular and start an IV drip of 10 drops/minute. 5. Isolate and free up the vagus nerve on each side and pass a loose thread under each nerve. Part 1. Effects of Inspired Gas Composition The following gas mixtures will be used. Each mixture will be made up in a 6-liter spirometer and transferred to a beach ball. The table shows how many milliliters of each gas to add to the spirometer to make each mixture. The beach ball is attached to the intake line of the tracheal cannula. Within 1 minute after attachment, the animal will be getting the new mixture. Allow at least 3-4 min for respiration to reach a new steady state. As soon as respiration is fairly steady, take your data record for that condition and return the animal to breathing air. Mixture # 1* %CO2 0.3 ml/6 L - %O2 20.9 ml/6 L - %N2 79 ml/6 L - 2 2 3 4 4 6 5 0 6 0 7 1.5 8 3 *normal atmosphere 120 240 360 0 0 90 180 21 21 21 14 7 14 7 1260 1260 1260 840 420 840 420 77 75 73 86 93 84.5 90 4620 4500 4380 5160 5580 5070 5400 Part 2. Effect of Increasing Dead Space Disconnect the transducer from the cannula, attach the dead space extension to the cannula, and reattach the transducer to the extension. You are increasing the dead space. Watch the animal and note changes in breathing pattern. Take a set of data. Remove the extension and return the setup to its original configuration. Part 3. Role of the Vagus Nerve in Control of Respiration This part will be done while the animal is breathing air. Whenever you see a significant change in respiration, take a set of recordings. 1. Take a set of recordings that will be used as the baseline for this part of the experiment. 2. Tie the right vagus near the caudal end of the exposed part. Cut the vagus below the tie, so the thread is now a handle for the cephalic end of the vagus. Do you see any changes in ventilation? Remember, you are cutting stretch receptor afferents from the lung and chest wall, but also thoracic chemoreceptors, baroreceptors, and parasympathetic fibers that run to the heart and upper abdominal viscera. 3. Stimulate the cephalic end of the vagus, using "burst" mode and pulses of 1 msec duration, frequency of 50/sec and about 0.5 V intensity or greater if necessary. Does respiration change? 4. Tie and cut the left vagus. Is there any further change in respiration? The animal now has no information about lung volume. Stimulate the cephalic end of the left vagus. Do you get the same effect as earlier? Part 4. Effect of Metabolic Acidosis 1. Take a record that will be used as the baseline for this part of the experiment. 2. Slowly inject 20 milliliters of 500 mM lactic acid via the IV line. Use your judgement about how fast to inject the lactic acid and whether to stop before the entire dose is given, since overdoing this may well kill the animal before any meaningful result occurs. You will probably want to take one record starting with the injection of the lactic acid, to show the onset of the response, and at least one more when the response is maximal, but use your judgement as this step progresses. ANALYSIS The computer will have two macros: RESP1 and RESP2. RESP1 integrates the primary flow rate data to give a record of the volume moved over time. As in the primary record, the plots show inspired volume as positive numbers and expired volume as negative numbers. For comparison, you can display the original record in channel 2. RESP2 clips off the negative flow values from the record and gives an integral of tidal volume over time. Each upward step in the resulting trace is the tidal volume of one cycle; the volumes of individual cycles accumulate over time so that the rise of the trace over a minute corresponds to minute volume. For each control and experimental condition, you will want to calculate the mean tidal volume, the minute volume and the respiratory rate. You can probably determine the rate using the fast Fourier transform utility routine; however, if the animal is breathing slowly, the Fourier transform nay not give an accurate rate and you will have to compute it by hand from the primary record. PREPARING YOUR REPORT For each experimental condition, calculate MV. Make a table that shows peak velocity, TV, RR and MV for each condition. For the gas mixtures, plot MV as a function of P CO2, including the value for air as zero. On a separate graph, plot MV as a function of PO2 for mixtures 1, 5 and 6. Using different symbols, plot the relation from 1, 7 and 8 on the same graph. You might get some additional insight into other aspects of respiratory control by plotting peak velocity, RR and TV as functions of P co2. Consider the following questions: 1. Is there a control system based on P co2? How do you know from your results? 2. Is there a control system based on P o2? How do your results indicate this? 3. Do the two systems interact? 4. Is the animal controlling only TV or RR, or is it separately varying TV and RR to minimize the work of breathing? 5. Explain the response to increased dead space. 6. How do your results fit what you know about lung and chest wall stretch receptors? Is MV maintained constant after vagotomy as compared to the value before vagotomy? How might this happen? 7. What happened when you made the animal acidotic and why? How does this response compensate for the decreased pH of plasma?