NAME: - Teacher Pages
advertisement

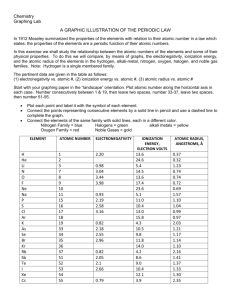
Name:_________________________Lab #:______Date:__________ Periodic Trends Lab Background: In this activity, you are going to discover some of the trends of the properties that exist on the modern periodic table. To do this, you are going to graph these properties. Objective: To define some of the properties of the element To graph the properties of the elements (use atomic numbers 1 through 36 only) To determine the general trends found on the periodic table Materials: graph paper, reference table, pencils Procedure: 1. On a piece of graph paper, graph the trend of first ionization energy (potential). The graph should have the atomic number on the x-axis and ionization energy on the y-axis. 2. Answer the questions about this graph and what conclusions you would draw. 3. On a piece of graph paper, graph the trend of electronegativity. The graph should have the atomic number on the x-axis and electronegativity on the y-axis. 4. Answer the questions about this graph and what conclusions you would draw. 5. On a piece of graph paper, graph the trend of atomic radius. The graph should have the atomic number on the x-axis and atomic radius on the y-axis. 6. Answer the questions about this graph and what conclusions you would draw. Analysis: Ionization Energy (Use graph on pg 174 to help answer questions) Create the graph as described in the procedure. 1. Define ionization energy. 2. What is the trend as you move down a group (column), increase or decrease? 3. What is the trend as you move across a period (row), increase or decrease? 4. On the representation of the periodic table below, draw arrows to represent increasing first ionization energy (potential). Electronegativity Create the graph as described in the procedure. 1. Define electronegativity. 2. What is the trend as you move down a group (column), increase or decrease? 3. What is the trend as you move across a period (row), increase or decrease? 4. On the representation of the periodic table above, draw arrows to represent an increasing electronegativity. Atomic Radius (Use graph on page 171 to help answer questions) Create the graph as described in the procedure. 1. Define atomic radius. 2. What is the trend as you move down a group (column), increase or decrease? 3. What is the trend as you move across a period (row), increase or decrease? 4. On the representation of the periodic table above, draw arrows to represent an increasing atomic radius. Summary: 1. Arrange the following atoms in order of increasing atomic radius: potassium, carbon, rubidium, iodine, fluorine, and lithium. 2. Explain why the atomic radius decreases across a period? 3. Arrange the following atoms in order of increasing ionization energy: lithium, oxygen, magnesium, strontium, chlorine, and tellurium. 4. Explain why ionization energy and electronegativity increases across a period? Properties of Elements (Data) Element Hydrogen Helium Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Sodium Magnesium Aluminum Silicon Phosphorous Sulfur Chlorine Argon Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine Krypton Ionization Energy (kJ/mole) 1312 2373 513 899 801 1086 1402 1314 1681 2081 496 738 577 789 1012 1000 1251 1520 419 590 633 659 651 653 717 762 760 737 746 906 579 762 947 941 1140 1351 Atomic Radius (pm) Electronegativity 37 32 134 125 90 77 75 73 71 69 154 145 130 118 110 102 99 97 196 174 144 132 122 118 139 125 126 118 117 120 120 122 122 117 114 110 2.2 1 1.6 2 2.6 3 3.4 4 0.9 1.3 1.6 1.9 2.2 2.6 3.2 0.8 1 1.4 1.5 1.6 1.7 1.6 1.9 1.9 1.9 2 1.7 1.8 2 2.2 2.5 3