Patient Group Direction for pharmacist supply of progestogen
advertisement
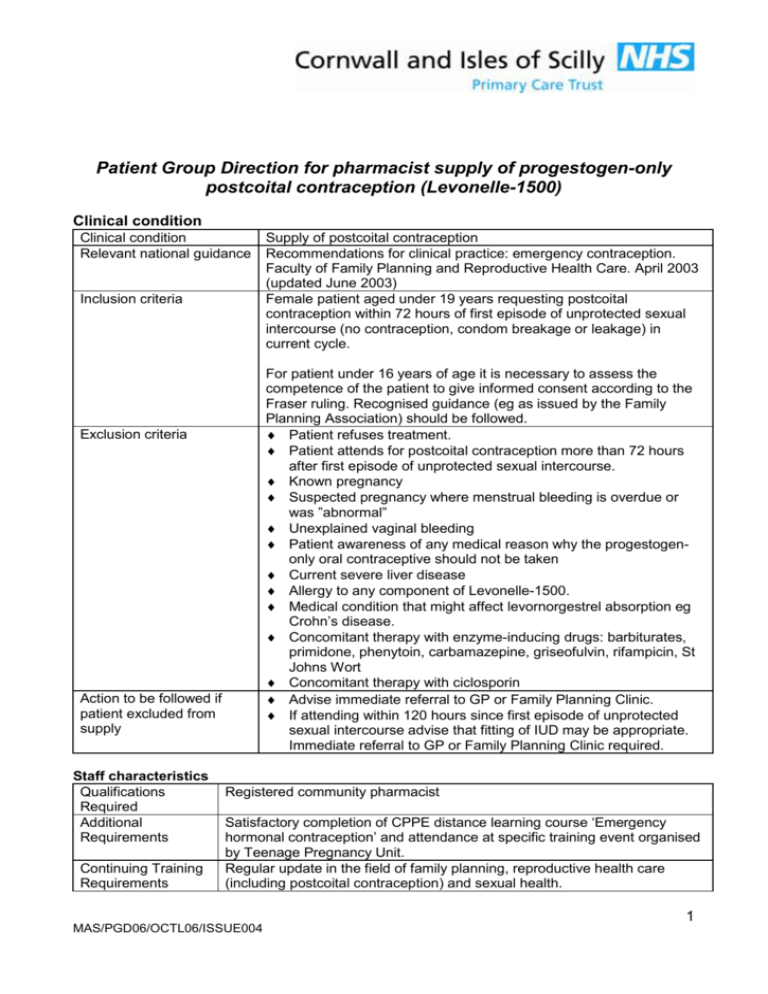
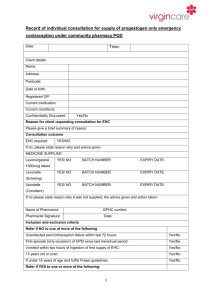
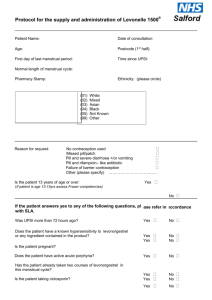
Patient Group Direction for pharmacist supply of progestogen-only postcoital contraception (Levonelle-1500) Clinical condition Clinical condition Relevant national guidance Inclusion criteria For patient under 16 years of age it is necessary to assess the competence of the patient to give informed consent according to the Fraser ruling. Recognised guidance (eg as issued by the Family Planning Association) should be followed. Patient refuses treatment. Patient attends for postcoital contraception more than 72 hours after first episode of unprotected sexual intercourse. Known pregnancy Suspected pregnancy where menstrual bleeding is overdue or was ”abnormal” Unexplained vaginal bleeding Patient awareness of any medical reason why the progestogenonly oral contraceptive should not be taken Current severe liver disease Allergy to any component of Levonelle-1500. Medical condition that might affect levornorgestrel absorption eg Crohn’s disease. Concomitant therapy with enzyme-inducing drugs: barbiturates, primidone, phenytoin, carbamazepine, griseofulvin, rifampicin, St Johns Wort Concomitant therapy with ciclosporin Advise immediate referral to GP or Family Planning Clinic. If attending within 120 hours since first episode of unprotected sexual intercourse advise that fitting of IUD may be appropriate. Immediate referral to GP or Family Planning Clinic required. Exclusion criteria Action to be followed if patient excluded from supply Staff characteristics Qualifications Required Additional Requirements Continuing Training Requirements Supply of postcoital contraception Recommendations for clinical practice: emergency contraception. Faculty of Family Planning and Reproductive Health Care. April 2003 (updated June 2003) Female patient aged under 19 years requesting postcoital contraception within 72 hours of first episode of unprotected sexual intercourse (no contraception, condom breakage or leakage) in current cycle. Registered community pharmacist Satisfactory completion of CPPE distance learning course ‘Emergency hormonal contraception’ and attendance at specific training event organised by Teenage Pregnancy Unit. Regular update in the field of family planning, reproductive health care (including postcoital contraception) and sexual health. MAS/PGD06/OCTL06/ISSUE004 1 Patient Group Direction for pharmacist supply of progestogen-only postcoital contraception (Levonelle 1500) Treatment Name of medicine Legal status Licensed use Dose of medicine Route of administration Frequency of dose Total number of doses supplied Nature of supply One tablet each containing levonorgestrel 1500mg (Levonelle-1500) POM The Summary of Product Characteristics for Levonelle-1500 states that Levonelle-1500 is not recommended in children. Very limited data available in women under 16 years of age. One tablet should be taken as soon as possible preferably within 12 hours and no later than 72 hours after unprotected intercourse. Oral One dose. The earlier in the 72-hour period the dose is given the greater the efficacy. It is therefore useful if the client takes the tablet in the pharmacy. One dose consisting of one tablet. Where possible encourage patient to take the tablet in the pharmacy in your presence. If the patient declines to do so, agree a time when it will be taken by the patient. Advice to be given to the patient Follow up treatment Records to be completed Audit trail If medication is to be taken away to take, the packet must be labelled with the name that patient provides, date of issue, directions for use, ‘pharmacy address’, ‘Keep Out of Reach of Children’ Discuss mode of action of postcoital contraception Discuss failure rate Counsel patient on possible side effects (nausea and vomiting, breast tenderness, headaches, dizziness, fatigue. Bleeding pattern may be temporarily disturbed). If vomiting occurs within three hours of taking the tablet, then contact doctor or Family Planning Clinic to obtain replacement tablet without delay. Identify package insert within the Levonelle-1500 pack. If patient attends because of missed pill, confirm that she knows how to proceed with remaining pill regime. Advise on need for ongoing and future contraception. Provide information on local family planning services. Provide information on sexually transmitted infections (STI) and local GUM services. Advise STI screen, particularly if recent change of sexual partner or two or more partners in the last twelve months. Advise patient to attend Family Planning Clinic or their GP if their next period is more than five days late or is unusual in any way, or, for those on the pill, if there is no bleed in the pill-free interval. Date of supply and dose regime used. Signature of pharmacist making supply. Batch number of Levonelle-1500. Completion of relevant check list All documents to be maintained and retained within the pharmacy for 3 years. MAS/PGD06/OCTL06/ISSUE004 2 Patient Group Direction for pharmacist supply of progestogen-only postcoital contraception (Levonelle 1500) Management PGD developed by Authorising Pharmacist Central Cornwall Primary Care Trust, Prescribing Team Jim Jiwa PEC Pharmacist Central Cornwall Primary Care Trust Signature of Authorising Pharmacist Date of PGD Date this PGD becomes due for review August 2008 Approved by: Central Cornwall PCT Nominated Doctor Central Cornwall PCT Pharmaceutical Adviser Name Dr Paul Travis Signature Bridget Sampson I This copy to be retained by the pharmacist MAS/PGD06/OCTL06/ISSUE004 3