1. Introduction. Age at death structure.
advertisement

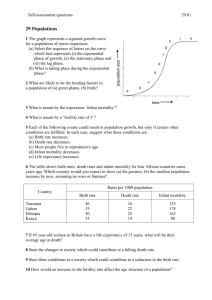
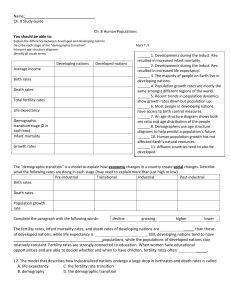
Chapter 12. Testing the estimates: age at Death structure. 1. Introduction 2. Results on resampling 3. Results on matches 4. Summary and conclusion 5. Discussion on use of age at death structures discsn Aging the bones agingbones Preservation babiesdisappear Methods for life table from bones methods Influence of fertility and increase fertnincr Attritional v catastrophic attritvscatstr Explanations for difference ancient and modern explanations No Tables Figures Figures References References 1. Introduction. Age at death structure. Age at death is another measure, influenced by fertility and mortality but in our case gathered by a different method than used to collect data on live age structure. Age at death data come from women’s reproductive history interviews, and from the “where are they now” interviews whereas live age structure came mainly from censuses or anthropometry data. Most importantly, scoring age at death structure does not rely on the “at risk” group that is so essential to estimating age specific mortality. We can match the observed Hadza age at death distribution to the distribution predicted by my population simulation from my estimates of age specific fertility and mortality. We can regard this match as another test of the accuracy of my estimates and of their stability during the last 50 years or so. I again use my resampling method to assess the matches. I also used Weiss’ (1973) and Paine’s (1989) method, described in chapter 11. Paine used his method in his campaign to convert archaeological demography to a deductive approach. Age at death distributions are the main data that archaeological and paleo - demographers have to work with. Since most of their populations are of modern Homo sapiens (all of the New World samples), and some are very recent, it makes sense, as Paine suggests, to try to see what population within the human range is most likely to have generated the excavated age at death distribution. I will discuss issues that arise when we compare living hunter-gatherer populations with populations from earlier times reported by palaeodemographers. 2. Resampling percent of deaths below age x. Nick Blurton-Jones Page 1 2/15/2016 I used resampling to establish the 95% confidence levels for % of deaths below age x, for all ages from 0 to 90. ure 12.3 shows what happens if we plot the observed and predicted values of % of deaths below age x for the Hadza. Observed age at death closely follows the prediction, although for males the observations fall a little below the predicted. Figure 12.3a. shows that the 95 percentiles of the observations on females follow closely to the values predicted with fertility increased by ten percent or decreased by ten percent. In the males (Figure 12.4), while the uppermost pair of lines run close to each other, the lower lines diverge quite clearly. The lower 95th percentile of the observations on males fall well below the level predicted if fertility was 10% lower than estimated. The median value falls below the median predicted value throughout adulthood. I have not shown graphs of predictions from altered mortality because there is so little to see! Even increasing or decreasing qx by 25% changes the expected cumulative age at death distribution very little. As Harpending and colleagues have warned (e.g Milner et al. 1989), age at death distributions are more sensitive to fertility and population increase than to mortality levels. This can also be seen in the Coale & Demeny tables. These data, like the observed age structure, suggest we may have slightly overestimated Hadza fertility, implying a slightly lower fertility than we estimated from reproductive interviews of women. But the Hadza pattern of emigration, with young women leaving to marry a “Swahili” and some of them returning later with some of heir children, deprives the population of some of its “fertility” and could account for this result. But the effect of this migration is actually very small. In Figure 12.5a we see that the Hadza female median observed values are, at most ages, between those of Ache and !Kung. For males (Figure 12.5b) the picture is complicated by the unusual pattern for the Ache. The proportion of deaths befalling Ache male infants and children is unusually low, even lower than for the near-stationary !Kung. Thus Hadza male percentage of deaths below age x, while running well above the !Kung trajectory, is at first also above the Ache. 3. Matching observed %of deaths in each age to predicted. Using Weiss’s method of testing the fit of observed structure to predicted structure we find the best fit of the observed age at death distribution is to the estimated fertility, models in which fertility is lower or higher give less good fits (Figure 12.1). When age at death structure is matched to a range of mortality levels the best fit is to a model in which mortality is reduced by 10% (Figure 12.2). Plotting the fits for each year shows that the method is primarily responding to variation in infant and early child mortality (not surprising since this is when most deaths occur). This may be more bad news for paleodemographers, although they often remove infants from their calculations, but in my context it again suggests a role for the Hadza pattern of female emigration. If Nick Blurton-Jones Page 2 2/15/2016 the number of births is lowered, the number of deaths will be also lowered. I estimated fertility from the women who were there, ignoring the women who were not there, but in the population simulation those women are generated, and so are their offspring. But they and their offspring are missing from the observations, so it will look as if the observations fit lower fertility or infant mortality than expected. But again, the effect of this migration on age at death structure is very small. 4. Summary and Conclusion. I used age at death distribution as another test of our population estimates. In the Hadza case fewer issues of data collection and treatment arise in measuring age at death distribution than arise in measuring the living age structure. Our source of data are the deaths in the population register, derived from women’s interviews and the “where are they now” interviews. The observations of age at death was independent of the observation of live age structure, and give us some hints about fertility (and less about mortality) without relying on our assessment of the number of people at risk at any age. The observational sources of the age structure and age at death structure were independent of each other. Like the age structure, age at death structure basically provides a good match to the predicted distribution. Deviations suggests that I may have slightly overestimated fertility and mortality. 5. Discussion. “No old people” again. Age at death distributions and paleodemography. A contrast is often made (for example by Weiss 1981, Austad 1997) between modern peoples, including ethnographically studied hunter-gatherers, whose populations include some numbers of vigorous 50-60 year olds, and prehistoric peoples, studied by palaeo - demographers using skeletal remains, among whom almost everyone was reportedly dead by 45. It is widely believed that in the past people suffered much higher mortality, had much lower life expectancy at birth, shorter potential lifespans, and fewer old people than contemporary hunter-gatherers. There is plenty of reason to doubt this belief. Because age at death structure is almost the only data that archaeologists can use, this is a good place to look again at the apparent conflict between data on longevity of living people, and conclusions about longevity in the past. In addition to estimating ages of skeletons, palaeo-demographers also sometimes estimate fertility from pelvic bone changes, and fertility estimates would increase the value of death distribution data. Nick Blurton-Jones Page 3 2/15/2016 Two issues of perspective need to be cleared up first. Most of the detailed archaeology and statistical analysis by paleo-demographers is done on quite recent populations. Especially in North America, palaeo-demographers have worked on populations from 1000 BC to say 1500 AD. There are written records from Old World and Asian populations from this time period, and these fit well within the range of mortalities of contemporary hunter-gatherers and rural third world populations, they are just like modern people. The difference is methodological, written people look like contemporary people, bone collections look like something else. Since the new world was populated rather recently (c.14,000 ya), and by modern Homo sapiens, we should be surprised if there were radical differences in their life histories. Paine and colleagues are surely right to suggest we use modern population models to test for similarity or differences between the bone assemblages and model modern human populations. But when we want to think about older populations, such as the Neolithic sites used by Weiss 1973: 96), or even older sites or peoples, such as Neanderthals (Trinkaus 1995), we may begin to wonder whether radical differences should be expected. But the size of the differences that are sometimes claimed is extraordinary. Why would the early farmers of Catal Huyuk have life expectancy at birth of 13.8 years (Weiss 1973 table A.24 from Angel 1969) while Hadza, !Kung and Ache have life expectancies over 30. If farming and city life was really so horrible, why put up with it? A second issue of perspective concerns what we mean by “old people”. Contrasts between modern and “primitive” populations, discussions of “old age in primitive times”, are sometimes merely an issue of perspective on what we call “old”. A researcher or clinician involved with old age in North America or Europe today works in a context of 90 year olds. We have no confident record of a 90 year old hunter-gatherer anywhere. At most there might have been, during recent fieldwork, one individual in each of our study populations who could have been, just possibly, somewhere close to being 90 years old. None of us allocated quite so high an age to these individuals. The same could be said for almost any rural, third world population. Our data on contemporary hunters and gatherers in no way contradict those who find useful provocation in the view that natural selection can have exerted little pressure against a disease of old age such as Alzheimer’s disease. Indeed the data support attempts to work out the implications of the much slower life cycle of the human primate (e.g. Finch & Sapolsky 1999, Sapolsky & Finch 2000). Hunter-gatherer researchers work in a different context, one of cooperative breeding (Hrdy 2009), cross-generation helpers (Hawkes et al 1998, wealth flows (Kaplan et al (1994), Lee (2003), and species differences (Hawkes 2006, Hill et al. 2001, 2007). We know there are no 90 year olds, and there are at best tiny numbers of 80 year olds. Here the issue is 50 and 60 year olds. How important are men and women of these ages for economically supporting their descendents? Why are 50-60 year old humans so strong and vigorous when compared to 50-60 year old Chimpanzees? For how long in human history and pre-history have there been some of these people alive and strong enough to acquire resources they can supply to children and grandchildren? How far back in our evolution did we develop age structures like humans and different from Chimpanzees? Nick Blurton-Jones Page 4 2/15/2016 There is much reason to believe that the difference between the archaeological populations and the observed or recorded populations is methodological and not real. Written records show populations like modern humans, bones from the similar periods show populations like some other species. An issue that is often mentioned, only to be promptly forgotten, is cultural variation in customs concerning disposal of the dead. Archaeologists hope to find a cemetery. Having found a good number of bones together, they are in danger of assuming it is an all inclusive cemetery like those of the developed world. Detailed suggestions about the methodological problems of “bone demography” can be found in the literature since its early days (see for example Angel 1969). They include: 1. Aging the bones 2. Preservation, loss of children and old people 3. Influences of population increase and fertility on age at death distributions 4. Methods for developing a life table from bone assemblies. 5. Attritional vs catastrophic assemblage – who gets found? 1. Aging the bones and the nature of the “standard” collections. Some of these samples are not so modern, and several were probably from very unhealthy people. The archaeological demographer has to suppose that the criteria of age described for a 50 year old 19th century factory worker represent all 50 year olds. It is possible that people who survive to 50 under the balanced, if limited, nutrition of the vigorous hunter-gatherer life have much younger looking bones than a 50 year old 19th century worker in a factory with no pollution or safety regulations. It is also possible that under hunter-gatherer conditions where only the fittest survive, the 50 year olds do have “younger” bodies than the average in a less fiercely selected (lower mortality) population. In the Spitalfields study of 18th century Londoners (Mollesen et al 1993), in which bone age estimates were compared with recorded, written ages of the same individuals, it was found that ages of many older people were seriously underestimated. Different methods of estimating ages from bones were compared by Wittwer-Backhofen et al. 2008. New methods are being sought and tested (e.g. Griffin 2009, DiGangi et al 2009, Cardoso & Henderson 2010). 2. Preservation, differential loss of children and elderly. Philip Walker and colleagues in an exceptionally important study, compared the written records of a mission in California with the bone assemblage excavated from its cemetery. Walker was able to demonstrate that the bones of small children and old people disappear faster than those of young adults. Archaeologists are alert to differences in quality of preservation but do we know how the loss of the extreme ages varies with state of preservation of those we find? Does judging preservation by the state of the young adult bones correctly assess the loss of bones of young and old? In working only with the best preserved do we risk other biases Nick Blurton-Jones Page 5 2/15/2016 in the samples? Samples from less organized societies may differ in many ways, disposal of corpses can differ by age and sex. 3. What do Age at death distributions show? In simple societies with high mortality, mortality level is a far weaker influence on age at death structure than fertility and population increase. Milner et al (1989) compared age at death distributions generated by fertility and mortality of !Kung and Yanomamo. They noted that the influence of fertility on age at death distribution was greater than that of mortality in this comparison. We can see the same thing by comparing various C&D models, looking at the age at death distributions. For example, we can roughly mimic Caspari & Lee’s 2004 comparison of the “Old/ Young” ratio of Neanderthal and upper Paleolithic samples. The O/Y ratio is the number of individuals in a bone assemblage (hence dead) aged over 30, divided by the number aged below 15. This was a promisingly conservative choice to avoid some of the problems of precise age estimation of archaeological material. A population experiencing C&D West 5 mortality (Howell’s best fit for the pre- 1960s !Kung) will show an OY ratio of 0.35 if the population is increasing at .020 p.a. but the ratio will climb to 1.84 if the population is decreasing at .01 pa. To match the 2.08 Caspari & Lee give for Early Upper Palaeolithic with a stationary population we have to raise the mortality level (reduce mortality) to C&D level 10. This takes us from an OY ratio of 1.04 for he stationary population at mortality level 5 (or an OY ratio of 0.52 in West 1) to a ratio of 2.16 for the stationary population at level 10. C&D model west 10 has a life expectancy at birth of 42.5 years (similar to the Datoga herder women in Borgerhoff Mulder et al. 1993) and at age 30 of a further 33.5 years. 4. Methods for developing a life table from bone assemblages. Those who read the paleodemographic record as showing very much higher mortality and shorter lives than recorded by historians or anthropologists apparently ignore the obstacles pointed out by palaeodemographers since at least Angel 1969: 430 “… gives no measure of death rates at any given age, a parameter for which one must know the number or percent alive at that age.” Methods used range from the nonexistent – simply assuming the age at death distribution is the same as the age distribution, via the notoriously bad - building the life table from the deaths alone, to the much improved and logically defensible. An important series of papers by Paine, Milner, Harpending and colleagues (e.g. Milner et al. 1989, Paine 1997, 2000) and by Konisgberg (eg. 2006) shows the difficulty of working from skeletal remains to an age structure, and ushers in a new perspective, proposing that bone assemblages be matched to age at death distributions predicted by a range of modern human population models. The starting assumption is that the archaeological collection was from regular humans, not markedly different from, for instance, the 326 populations used to build the C&D models. While some have incorrectly assumed that an age at death distribution is the same as an age structure, others have directly derived lx from a death distribution. The Nick Blurton-Jones Page 6 2/15/2016 assumptions that must lie behind this do not seem widely appreciated. The method can possibly be defended in the case of a historical cemetery in a small rural village with no emigration. Suppose everyone who lives in the village dies there and is buried in the village (church) cemetery, and this continues for many years (say ninety years or more). Then the longer lived contemporaries of the toddler who died aged 2, will be found somewhere in the cemetery with stones marked (or entered in the parish register) with the year in which they were born and died. He may have a cousin who died aged 8, and a brother who lived to 75. Everyone is in there, no one escaped. In this case, all the people who lived through their first and second years died at ages greater than 2 and will be found. Then the number of later deaths can be safely used as the at risk group for year one and year two. All those who died at ages greater than 75 can be used as the at risk group for the 75 year olds, and so on. The procedure is heavily dependent on there being no emigration, good recording, no converts to a competing religion buried somewhere else that we do not know about. If there was emigration, commonly to the cities where recording is more diffuse and cemeteries more numerous, we lose data on the at risk group and on the ages at death of emigrants. If we wish to characterize the village, this may be no problem. If we wish to characterize survival schedules of the species, nation, tribe, we have lost essential information. These conditions are more restrictive than some might have supposed. Historians describing the demography of northern, Christian populations are in good shape (they are usually able to use parish records which include birth dates and allow an estimate of emigration). Almost everyone else who tries to use this method elsewhere faces problems. For example, Walters’ (2008) describes several special problems of church records in northern Tanzania (for example, how the church did or did not record second wives). Next, let’s think about using this method on a contemporary, observed population, quite tempting in view of the difficulties of being sure of the at risk sample. Howell (1979:Table4.4 and pages 87-90) showed how this might be done, suggesting “imagine for a moment…that the !Kung had established a graveyard in 1963”. Analyzing just the !Kung who died between 1963 and 1974 (her Table 4.4) she developed an lx curve and found a life expectancy of 34.57 years, rather close to her estimate for a previous time period. She then remarks: “Demographers are generally wary of the validity of computing mortality from a collection of deaths … because the denominator of the qx measures, conceptually, is the number of people at risk of dying in the living population” (just like the palaeodemographer Angel cited above). When she calculated survivorship for the 1963-74 period by the usual demographic method the life expectancy was over 50.1 years (Howell 1979 Table 4.6). She then proceeds to simulation runs to assess the reliability of this surprisingly high figure. If we compare these two methods for the Hadza we again get radically different results. Life expectancy at birth calculated on 418 deaths of males and females is 20.7, well below my observed 32.7. Why does this happen? In a contemporary population where we collect data on deaths in the past few years, each dead person has contemporaries who are still alive. Even the oldest dead people may have one or two age mates who outlive them. Among those who died in mid life or earlier, there will be many who outlived them and still have not arrived in our list of ages at death. It is extremely unlikely that a field anthropologist Nick Blurton-Jones Page 7 2/15/2016 could collect ages at death for a sample who have no surviving contemporaries. An anthropologist’s age at death collection cannot meet the “English village church yard” criteria. If one assumes that the archaeological specimens are a random selection from those who died in the period covered by the record, then they surely should represent an age at death distribution. It seems impossible to make a case for taking them as age structures, representative of living individuals. 5. Attritional versus catastrophic assemblages. An important development has been the recognition that some assemblages result from some sudden, large scale catastrophe, a raid or epidemic, while others represent the gradual accumulation of the results of “day to day” attrition. A catastrophic assemblage has some chance of resembling the living age structure (everyone was struck down indiscriminately). An attritional assemblage, like any age at death distribution is a result of an interaction between age structure (the number of people in each age) and the rate of deaths at each age. By simulating population crashes Keckler (1997) was able to show how catastrophic assemblages might be recognized. Catastrophic events may be responsible for many of the well studied bone assemblages with unusual age at death distributions. The Hadza can illustrate the difficulty of finding a good “attritional” collection. In such a population the chance of finding a collection of skeletons that represent the normal age at death distribution is very slender. There are on average 28 deaths per year. In the average census there were 25 camps. We can expect just over 1 death per year per camp. Camps move quite frequently, 6.5-9 times per year according to Marlowe (2010: 263). Let’s call it 8 moves per year. The chance of a death at a particular camp site is 1/ 8 per year, one every 8 years if people return to the exact same spot. If an adult dies, people leave the location. People often camp again in the same general area but seldom at exactly the same site as one of their previous camps. But eventually people may lose track of exactly where they camped and in some favored locations there is some chance of camps being superimposed on previous camps. Then, after some years, there is some chance of another corpse being left behind at or near (within 100 meters) of a previous corpse. The amount of time needed to accumulate a significant sample of remains would be truly immense. Let’s play with these numbers. Suppose people forget where someone was buried or left behind, or no longer care, after 10 years (too soon), 20, or 30 (perhaps too long?). Then if someone dies at this camp, a 1 in 8 chance, a second corpse may be left nearby in 80 years, or 160, or 240 years. A collection of ten corpses would take between 800 and 2400 years to develop in one place. There can be very few sizable attritional assemblages of mobile hunter gatherers out there to find. In contrast, if there are violent raids, or serious epidemics, and a large proportion of the people in camp died, the archaeologist has only to chance upon a raided or infected camp to find a useful sample of remains. This seems to me to imply that any sizeable Nick Blurton-Jones Page 8 2/15/2016 collection of remains of mobile hunter – gatherers should a priori be assumed to represent a catastrophe, not the death distribution of a stable population. Explanations for differences between ancient and modern. Those who believe that the longevity of all observed contemporary and historically known populations is very new, its antiquity disproved by the archaeological samples, have offered a variety of explanations for the supposedly recenjt change. Modern life is easier than prehistoric life, and modern medicine greatly reduces morbidity and mortality. It undoubtedly does. But does it account for the mortality and age structure seen among modern third world populations before modern medicine was widely available? Like !Kung in the 1950s1960s, and Ache in the “forest period” before contact, the Hadza, with minimal contact with modern medicine, who all show a "normal” human mortality and age at death distribution. They also ignore the fact that some of the archaeological populations are more recent than some of the historical populations. Others attribute the difference to “culture”, as if the people of Libben, or Catal Huyuk had no culture. However, somewhere back in our evolution our ancestors probably lived no longer than Chimpanzees. Judging by size, Australopithecines had a life history quite similar to Chimpanzees. Indeed the Chimpanzee e15 in Hill et al 2001 (15.4 years for females and 14.2 for males) closely matches the e15 estimated by Weiss 1973 Table A20 from the Australopithecine life table bravely created by Mann (1968) from fossils. But the lx estimated from Australopithecine fossils is far below the lx observed among Chimpanzees. When did our ancestors begin to live significantly longer than chimpanzees? Several schedule offerings are in the literature. O’Connell, Hawkes and I (1999) argued that a major change in life history accompanied the origin of Homo erectus. We suggested that the relatively sharp increase in size with H erectus should imply later maturity which is expected to follow from lower mortality and longer lifespans. Others have argued for longer life and lower mortality arriving with Homo sapiens, or even with only the more modern forms of our species (e.g. Upper Palaeolithic Europe Caspari & Lee (2004). These leave us without an explanation for the similar size of erectus and sapiens. Since age at maturity or first reproduction is well correlated with life span across taxa, the investigation of speed of development and age at maturity has implications for dating the arrival of human longevity. So far, these seem to favor development of Homo erectus individuals quite a bit faster than seen among modern humans (e.g Dean & Smith 2009). A simple taxonomic parsimony argument appeals to me as a way to set a maximally recent date. Characters found in all representatives of the species are likely to date from the origin of the species but may or may not also have been present in its extinct ancestors. The alternative is independent evolution of the character in different parts of the range, and a subsequent filling in by the supposedly advantageous character. Imagine the capacity for language evolving independently everywhere in the world. Much simpler to suppose it originated at the latest with Homo sapiens. Nearly all living humans have a similar life table, one that differs quite markedly from Chimpanzees, Nick Blurton-Jones Page 9 2/15/2016 mainly in adult survival. The real exceptions are people in industrial societies with a convex lx curve, with a great majority of deaths in the 80s. Demographers know a great deal about their history. It is truly recent. So it seems most likely to me that the basic human life table evolved before the expansion out of Africa. Since that expansion came from a relatively small part of our range within Africa, and well after the divergence of Khoisan speakers, the human life table likely arose at least as far back as the origin of modern Homo sapiens. The argument from size implies a similar life table for Neanderthals and any other large archaic Homo. The description of the occasional very old Neanderthal widens the taxonomic range. My taxonomic parsimony argument then brings us back to our 1999 suggestion, that long lifespans began with Homo erectus. A new approach to determining when human lifespans lengthened is beginning to make its presence felt. The field will no longer be a simple contrast between the findings of anthropologists and archaeologists. The genes responsible for variation in lifespan are becoming known. And eventually their times of origin will be estimated, with their characteristically wide confidence intervals. Already, Finch & Stanford (2004) in their paper “Meat-adaptive genes and the evolution of slower aging in humans” outline contributions of the ApoE3 allele to longevity by reducing inflammatory responses, increasing bone strength, and adjusting cholesterol responses to dietary fat. Citing Fullerton et al. (2000) they report the ApoE3 allele as dating from about 311 kya (176579 kya) with an expansion around 226 kya. They comment that this dates the spread of apoE3 before expansion out of Africa and perhaps allows its presence in Neanderthals and earlier Homo in Africa. They tentatively implicate a few other genes in the process, with similarly timed histories. Other genes affecting vigor and longevity are found in mitochondria and among the nuclear genes controlling them (e.g. Wallace 2010). When the histories of these and other genes that increase lifespan are developed, we may have clusters of origin times for changes in longevity that can be attached to the fossil record. Figures for Age at Death chapter 12. 12.1. Dissimilarity index for deaths in age for different fertility levels. 12.2. Dissimilarity index for deaths in age for different mortality levels 12.3 Percent of deaths below age x, females 12.4 Percent of deaths below age x, males 12.5 a & b. Percent of deaths below age x. Comparing Hadza, ache, and !Kung. Figure 12.1. Match of observed Hadza age at death structure to structure predicted by different levels of fertility. Nick Blurton-Jones Page 10 2/15/2016 Scatterplot of fertdiss vs fertadj2 0.28 0.26 0.24 fertdiss 0.22 0.20 0.18 0.16 0.14 0.12 0.10 -30 -20 -10 0 10 20 fertadj2 Minitab-for-plotting-Weiss-11-9-3.mpj Nick Blurton-Jones Page 11 2/15/2016 Figure 12.2. Match of observed Hadza age at death structure to structure predicted by different levels of mortality. Scatterplot of dissdedin vs mortadj2 0.1475 0.1450 dissdedin 0.1425 0.1400 0.1375 0.1350 -30 -20 -10 0 mortadj2 10 20 30 Minitab-for-plotting-Weiss-11-9-3.mpj Nick Blurton-Jones Page 12 2/15/2016 Figure 12.3. Females. Percent of deaths below age x. The central lines with markers are the observed and predicted figures. The light orange lines show the predicted value when 10% of the ASF is added to or subtracted from the input to the population simulation. The light pink lines show the 95% confidence limits for the observations obtained from resampling. 120 100 % dead by age x 80 f +10% ASF f -10% ASF obs f hi obs f lo obs f median %fdedbelowASF0 60 40 20 0 0 10 20 30 40 50 60 70 80 90 100 age Nick Blurton-Jones Page 13 2/15/2016 Figure 12.4 Males. Percent of deaths below age x. The central lines with markers are the observed and predicted figures. The dark blue lines show the predicted value when 10% of the ASF is added to or subtracted from the input. The light blue lines show the 95% confidence limits for the observations obtained from resampling. 120 100 % deaths below age x 80 m m m m m m 60 +10% ASF - 10% ASF obs hi obs lo obs med pred ASF0 40 20 0 0 10 20 30 40 50 60 70 80 90 100 age Nick Blurton-Jones Page 14 2/15/2016 Figure 12.5a. Female age at death, 95 percentiles of observations plotted alongside Ache and !Kung age at death. Ache computed from female deaths reported in Hill & Hurtado Table 6.1 ninth column. 120 100 % of deaths below age x 80 Ache f ded W5 r0 f obs lo f obs hi 60 40 20 0 0 10 20 30 40 50 60 70 80 90 100 age x Nick Blurton-Jones Page 15 2/15/2016 Figure 12.5.b. Male age at death, 95 percentiles of observations plotted alongside Ache and !Kung age at death. Ache computed from male deaths reported in Hill & Hurtado Table 6.1 ninth column. 120 100 % of deaths below age x 80 Ache male W5r0 male m ded lo m ded hi 60 40 20 0 0 10 20 30 40 50 60 70 80 90 100 age x Nick Blurton-Jones Page 16 2/15/2016 References Angel, J. L. 1969. The bases of Paleodemography. American Journal of Physical Anthropology 30:427-438. Austad, S. 1997. “Postreproductive survival,” in Between Zeus and the Salmon: the Biodemography of Longevity. Edited by K. W. W. C. E. Finch. Washington DC: National Academy Press. Borgerhoff Mulder, M. 1993. Demography of pastoralists: preliminary data on the Datoga of Tanzania. Human Ecology 20:1-23. Cardoso, F. A., and C. Y. Henderson. 2010. Enthesopathy formation in the humerus: data from known age-at-death and known occupation skeletal collections. American Journal of Physical Anthropology 141:550-560. Caspari, R., and S.-H. Lee. 2004. Older age becomes common late in human evolution. Proceedings of the National academy of Sciences 101:10895-10900. Coale, A., and P. Demeny. 1983. Regional Model Life Tables and Stable Populations. New York: Academic Press. Dean, M. C., and B. H. Smith. 2009. “Growth and development of the Nariokotome youth,” in The first humans: origin and early evolution of the genus Homo, vertebrate paleobiology and paleoanthropology. Edited by f. E. Grine and e. al., pp. 101-: Springer Science. DiGangi, E. A., J. D. Bethard, E. H. Kimmerle, and L. W. Konigsberg. 2009. A new method for estimating age-at-death from the first rib. American Journal of Physical Anthropology 138:164-176. Finch, C., and R. Sapolsky. 1999. the evolution of Alzheimer's disease, the reproductive schedule, and apoE isoforms. Neurobiology of Aging 20:407 - 428. Finch, C. E., and C. B. Stanford. 2004. Meat-adaptive genes and the evolution of slower aging in humans. The Quarterly Review of Biology 79:3-50. Fullerton, S. M., A. G. Clark, K. M. Weiss, D. A. Nickerson, S. A. Taylor, J. H. Stengard, V. Salomaa, E. Vartiainen, M. Perola, E. Boerwinkle, and C. F. Sing. 2000. Apolipoprotein e variation at the sequence haplotype level: implications for the origin and mainatenance of a major human polymorphism. American Journal of Human Genetics 67:881-900. Griffin, R. C., A. T. Chamberlain, G. Hotz, K. E. H. Penkman, and M. J. Collins. 2009. Age estimation of archaeological remains using amino acid racemization in dental enamel: a comparison of morphological, biochemical, and known ages-at-death. American Journal of Physical Anthropology 140:244-252. Hawkes, K. 2006. “Life History theory and human evolution: a chronicle of ideas and findings,” in The evolution of human life history. Edited by K. Hwkes and R. R. Paine, pp. 45-93. Santa Fe: School of American Research Press. Hawkes, K., J. F. O'Connell, and N. G. Blurton Jones. 1989. “Hardworking Hadza Grandmothers,” in Comparative Socioecology. Edited by V. S. a. R.Foley, pp. 341-366. Oxford: Blackwell. Hawkes, K., J. F. O'Connell, N. G. Blurton Jones, H. Alvarez, and E. L. Charnov. 1998. Grandmothering, menopause, and the evolution of human life histories. Proceedings of the National Academy of sciences, USA 95:1336-1339. Nick Blurton-Jones Page 17 2/15/2016 Hill, K., C. Boesch, J. Goodall, A. Pusey, J. Williams, and R. Wrangham. 2001. Mortality rates among wild Chimpanzees. Journal of Human Evolution 40:437-450. Howell, N. 1979. Demography of the Dobe Area !Kung. New York: Academic Press. Hrdy, S. B. 2009. Mothers and Others. Cambridge, Mass.: Harvard University Press. Kaplan, H. 1994. Evolutionary, and Wealth flows theories of Fertility. Empirical tests and new models. . Population and Development Review 20:753-791. Keckler, C. N. W. 1997. “Catastrophic mortality in simulations of forager age-at-death: where did all the human go?,” in Integrating Archaeological Demography: Multidisciplinary Approaches to Prehistoric Populations, Center for archaeological Investigations Occasional Papers No 24. Edited by R. Paine, pp. 205-227. Carbondale IL: Southern Illinoios university Press. Konisgberg, L., and N. P. Herrman. 2006. “The Osteological Evidence for Human Longevity in the Recent Past,” in The Evolution of Human Life History. Edited by R. R. P. Kristen Hawkes , pp. 267-306. Santa Fe, Oxford: School of American Research Press, James Currey. Lee, R. D. 2003. Rethinking the evolutionary theory of aging: Transfers, not births, shape senescence in social species. Proceedings of the National academy of sciences 100:9637-9642. Mann, A. E. 1968. The paleodemography of Australopithecus., University of California. Marlowe, F. W. 2010. The Hadza Hunter-Gatherers of Tanzania. Origins of Human Behavior and Culture. Berkeley, Los angeles, London: University of California Press. Milner, G. R., D. A. Humpf, and H. C. Harpending. 1989. Pattern matching of age-atdeath distributions in palaeodemogrpahic analysis. American Jornal of Physical Anthropology 80:49-58. Molleson, T., Cox, M. et al. 1993. The Spitalfields Project. York: Council for British Archaeology. O'Connell, J. F., K. Hawkes, and N. G. Blurton Jones. 1999. Grandmothering and the evolution of Homo erectus. Journal of Human Evolution 36:461-485. Paine, R. 2000. If a Population Crashes in Prehistory, and there is no Paleodemographer there to hear it, does it make a sound? American Journal of Physical Anthropology 112:181-190. Paine, R. R. 1989. Model life table fitting by maximum likelihood estimation: a procedure to reconstruct Paleodemographic characteristics from skeletal age. American Journal of Physical anthropology 79. Paine, R. R. Editor. 1997. Integrating archaeological demography: multidisciplinary approaches to prehistoric population. Vol. 24. Center for Archaeological Investigations, Occasional Papers. Carbondale, IL: Center for Archaeological Investigations. Sapolsky, R. M., and C. E. Finch. 2000. Alzheimer's disease and some speculations about the evolution of its modifiers. Annals of the New York Academy of Sciences . Walker, P. L. J., J.R., Lambert, P.M. 1988. Age and Sex biases in the Preservation of human skeletal Remains. American journal of Physical Anthropology 76:183-188. Wallace, D. C. 2010. Mitochondrial DNA mutations in disease and aging. Environmental and Molecular Mutagenesis 51:440-450. Nick Blurton-Jones Page 18 2/15/2016 Walters, S. L. 2008. Fertility, Mortality and Marriage in Northwest Tanzania, 1920-1970: a Demographic study using parish registers. Doctor of Philosophy, Cambridge University. Weiss, K. M. 1973. Demographic Models for Anthropology. American Antiquity 38:Part 2. Weiss, K. M. 1981. “Evolutionary Perspectives on Human Aging,” in Other Ways of Growing Old. Edited by P. T. A. S. Harrell, pp. 25-58. Stanford CA: Stanford University Press. Wittwer-Backhofen, U., j. Buckberry, A. Czarnetzki, S. Doppler, G. Grupe, G. Hotz, A. Kemkes, C. s. Larsen, D. Prince, J. Wahl, A. Fabig, and S. Weise. 2008. Basics in Paleodemography: a comparison of age indicators applied to the early medieval skeletal sample of Lauchheim. American Journal of Physical Anthropology 137:384-396. Nick Blurton-Jones Page 19 2/15/2016