SUPPLEMENTARY INFORMATION

SUPPLEMENTARY INFORMATION
Tissues and cells. For murine DCs generation, marrow cells were dispersed by vigorous pipetting and cultured in RPMI-1640 supplemented with penicillin (100 μg /ml), streptomycin (100 U/ml), L-glutamine (2 mM), 2-mercaptoethanol (50 M; Sigma-
Aldrich, St. Louis, MO) and 10% heat-inactivated FBS in the presence of 20 ng/ml of recombinant mouse granulocyte-macrophage colony-stimulating factor (GM-CSF, 315-
03, Peprotech Inc., Rocky Hill, NJ) for 8 days. GM-CSF was replenished on days 3 and
6. In some experiments, maturation was induced by culturing the cells for 2 days in the presence of 10 ng/ml GM-CSF, 20 ng/ml mouse tumor necrosis factor alpha (TNF ,
315-01A, Peprotech) and 1 g/ml bacterial lipopolysaccharide (LPS from E. coli , serotype 0111:B4, L2630, Sigma).
For monocyte generation, bone marrow cells were obtained as above and plated at
1 x 10 5 cells/well in RPMI 1640 10% FBS medium containing 10 ng/ml macrophage colony stimulating factor (M-CSF, G0282, Sigma). M-CSF was replenished after 3 days of incubation. Cells were used for different studies after an additional 3 days.
Human adherent monocytes were obtained through leukapheresis. and cultured for 7 days in AIM-V media (Invitrogen) supplemented with 800 IU/ml human GM-CSF
(Immunex, Seattle, WA) and 500 IU/ml human interleukin-4 (IL-4; R&D Systems,
Minneapolis, MN) to generate immature DCs. On day 7, cells were collected, counted by trypan blue exclusion, and cultured for an additional 4 days at 5 x 10 5 cells/ml in AIM-V media supplemented with 3% heat-inactivated filtered human AB serum
(BioWhittaker/Cambrex, East Rutherford, NJ), 1000 IU/ml GM-CSF, and 1000 IU/ml IL-
4. DC maturation was induced by adding 20 ng/ml TNF-α (R&D Systems) on day 7 and an additional 10 ng/ml TNF-α on day 9. Cells were harvested for phenotypic and functional analysis on day 11.
Tumors.
For flank injections, a single-cell suspension of ID8-VEGF cells was prepared in PBS mixed with an equal volume of cold Matrigel™ (BD Biosciences) at 10 mg/ml. A total volume of 0.5 ml containing 7 × 10 6 cells was subcutaneously injected into one flank of C57BL/6 mice. Tumors were detectable two weeks later and were measured weekly using a Vernier caliper. Tumor volumes were calculated by the formula V= ½ L x
W 2 , where L is length (longest dimension) and W is width (shortest dimension). For i.p. injections, 1 × 10 7 ID8-VEGF cells were suspended in a total volume of 0.7 ml PBS and inoculated i.p. into the mice.
Immunostaining. Immunohistochemical staining was performed as previously described using the avidin-biotin-peroxidase method. Sections were fixed in cold acetone for 10 minutes, pretreated with 3% H
2
O
2
for 20 min to block endogenous peroxidase activity and incubated in matched normal sera (Vector Laboratories). Biotinylated rat anti-mouse
CD11c (BD Pharmingen) was diluted at 1:100. Anti-human CD11c (S-HCL-30), CD83
(HB15a), CD14
(M5E2)
(all BD Pharmingen), CD1a (sc-7091) (Sta. Cruz Biotech.),
CD68 (KP1) (DAKO, Carpinteria, CA) were diluted 1:100. The Vectastain ABC kit was applied as described by the manufacturer (Vector Laboratories). Sections were counterstained with Gill’s hematoxylin (Vector Laboratories). Images were acquired
through Cool SNAP Pro color digital camera (Media Cybernetics, Carlsbad, CA). Ten different fields for each sample at ×400 magnification were evaluated for cell counting.
For immunofluorescense analysis, sections were sequentially incubated with antimouse CD16/32 antibody (1:100 dilution) and PE-anti-mouse CD14 or NK1.1 antibodies
(1:100 dilution; rmC5-3, BD Pharmingen). Sections were counterstained with 4',6'diamidino-2-phenylindole hydrochloride (DAPI) before being inspected under the fluorescent microscope.
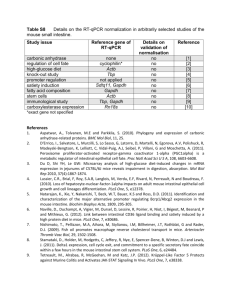
Real-Time Quantitative Reverse Transcription-PCR.
The following mouse primers were used: interferon gamma (IFN-γ) F 5’-TGG AGG
AAC TGG CAA AAG GA-3’, IFN-γ R 5’-TGT TGC TGA TGG CCT GAT TG-3’; IL-4
F 5’-TCT TTC GGG CTT TTC GAT GC-3’, IL-4 R 5’-CCA GGA AGT CTT TCA GTG
ATG TGG-3’; MIG F 5’-CAA GCC CCA ATT GCA ACA AA-3’, MIG R 5’-TCC GGA
TCT AGG CAG GTT TGA-3’; IP-10 F 5’-TGC TGG GTC TGA AGT GGG ACT-3’,
IP-10 R 5’-AAG CTT CCC TAT GGC CCT CA-3’. For human samples the following primers were used: MIG F 5’-GAC CTT AAA CAA TTT GCC CCA AG-3’, MIG R 5’-
CAC ATC TGC TGA ATC TGG GTT TA-3’; IP-10 F 5-TAT TTC CCT CAC CTT TCC
CAT CT-3’, IP-10 R 5’-TCT GAT AAA CCC CAA AGC AGA AA-3’. We normalized the cDNA load to mouse/human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) with primers GAPDH F 5'-CCT GCA CCA CCA ACT GCT TA-3' and GAPDH R 5'-
CAT GAG TCC TTC CAC GAT ACC A-3'. Data were expressed as relative units respect to 10 6 GAPDH mRNA molecules. Molecules were considered to be present if more than five copies of mRNA were detected for every 10 6 copies of GAPDH mRNA.