doc
advertisement

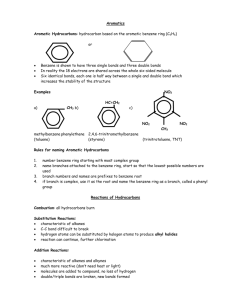
1 ORGANIC CHEMISTRY The study of compounds with carbon - An organic compound has carbon - A inorganic compound does not Common elements in organic chemistry are Carbon - 4 valence e- has 4 bonds Hydrogen - 1 valence e- has 1 bond Oxygen - 6 valence e- has 2 bonds Nitrogen - 5 valence e- has 3 bonds Carbon and other elements can link together in many different ways to form organic compounds. HYDROCARBONS - compounds with carbon and hydrogen - hydrocarbons are classified according to whether or not they have multiple bonds 1. Alkanes suffix -ane All bonds in carbon chain or ring are single bonds 2. Alkenes suffix -ene Hydrocarbon has one or more double bonds between carbon atoms 3. Alkynes suffix -yne Hydrocarbon has one or more triple bonds between carbon atoms Nomenclature of Alkanes 1. Need to count longest continuous chain of carbons 2. Number of carbons gives compound’s prefix 1 meth 6 hex 2 eth 7 hept 3 prop 8 oct 4 but 9 non 5 pent 10 dec 3. Suffix is –ane. Examples: butane C C C heptane C C C C C C C C 2 4. Carbon chains branching off main chain are given numerical prefix and suffix –yl. 5. The main chain carbon where branching occurs is given the lowest possible number. 6. To write name, put pendant groups in alphabetical order indicating main chain carbon number and branch’s prefix. Example H H H C H H H C H H H H C C C C C H H H H H H Note that longest continuous carbon chain has 6 atoms; therefore this alkane is a hexane. Also the hydrocarbon has a one carbon branch (a methyl branch). Now we need to determine from what carbon the methyl branch is branching. We count the carbons on the main chain from either end. H H 1 H H H C H C H H 2 H C H 6 H H H H H H C H H 5 3 H C 4 C 5 C 6 C C H H C C C 4 H H H H H H H C 3 H C 2 H H 1 H The methyl branch is coming from either carbon 3 or carbon 4. We need to use the lower number. Therefore, the name of the hydrocarbon is 3-methylhexane. 3 Example H H H H H H H H C C C C C C C H H H H H H C H C H H H H C H H H Note that longest continuous carbon chain has 8 atoms; therefore this alkane is an octane. Also the hydrocarbon has a two-carbon branch (an ethyl branch). Now we need to determine from what carbon the ethyl branch is branching. We count the carbons on the main chain from either end. H 1 H H 2 H 3 H 4 H H H C C C C H H H H H 8 5 C C C C H H H H H H H H H H 7 H 6 H 5 C H C C H H H H 4 C C C C C H H H H H H C 6 7 H 3 C H 2 8 H C H H C H 1 H H The ethyl branch is coming from either carbon 5 or carbon 4. We need to use the lower number. Therefore, the name of the hydrocarbon is 4-ethyloctane. 4 Example H H H H 1 H 2 C 3 C 9 H H 6 C 6 H H 5 C 7 H H 4 C 8 H H H C 7 C 5 H H 8 C 4 9 C 3 C C 2 H H H H H 1 H H H C H H The longest continuous carbon chain has 9 atoms; therefore this alkane is a nonane. This alkane has a methyl branch and an ethyl branch. By numbering the branch point from either end of the longest chain we can name the alkane either 6-ethyl-3-methylnonane or 4-ethyl-7-methylnonane. The lower set of numbers is correct; therefore, the proper name is 6-ethyl-3-methylnonane. Example: Draw a structure for the alkane: 2,2-dimethylpentane First we need to recognize that since that the compound is a pentane, it has a carbon chain of 5 atoms. H H H H H H C C C C C H H H H H H The term, dimethyl, means that the hydrocarbon has 2 one-carbon branches. The numbering tells us that both branches are on the second carbon. In drawing the methyl branches, you have a free choice. You may number the carbon atoms on the chain from the left or right. H H H H H C H H H H C C C C C H H C H H H H H H H H H H H C H H C C C C C H H H H C H H H H 5 We can be more sophisticated in drawing organic structures by drawing only bonds between the carbon atoms. Our sophisticated knowledge of hydrocarbons allows us to include hydrogens if we want; however, when discussing complicated alkanes, sometimes ignoring the hydrogens adds clarity. These structures are called line structures. In a line structure, hydrogens are never explicitly drawn; thus, carbon atoms are found at the corners (or kinks) in the chain as well as at the end of a line. 3-methylhexane 6-ethyl-3-methylnonane Types of carbons in a chain Primary – one other carbon attached Secondary – two other carbons attached Tertiary – three other carbons attached 4-ethyloctane 2,2-dimethylpentane 6 Structural isomers Two molecules that have the same chemical formula, but different molecular structures are structural isomers. Example C2H6O H H H C C O H H H Ethanol (also CH3CH2OH) - Ethanol has 62% of the energy content of octane (2,2,4-trimethylpentane) H H H C O C H H H Dimethylether (also CH3OCH3) - one of the first medical anesthetics Nomenclature of alkenes and alkynes Same as alkanes except - must use different suffix - must indicate where double (triple) bond is in carbon chain. C C C C 1-butene - give number to lowest possible carbon - sometimes need to indicate geometrical isomer C C C C C C C C If branches are on same side of double bond, then molecule has cis geometry. If branches are on opposite sides of double bond, then molecule has trans geometry. cis-2-butene trans-2-butene Example: What are the names of the following structures? C C C C C C C C C C C C C C 2,5-dimethyl-cis-3-octene 4-methyl-2-pentyne C C 7 Nomenclature of cyclic hydrocarbons C C C C C C cyclopropane C C C cyclobutane C C C C C C C C C C C C 1-ethyl-2-methylcyclopentane cyclohexene C C C C C 1-methylcyclobutene AROMATIC HYDROCARBONS 1,3,5-cyclohexatriene is an especially stable alkene. This compound is referred to as benzene. C C C C C C C C C C C C Resonance makes benzene very stable. To illustrate better resonance, the benzene molecule is often written as C C C C C or C Benzene is carcinogenic; therefore, it is no longer used as an industrial solvent. Benzene is an important part of many strong polymers. 8 Other aromatic compounds can come from linking benzene rings to each other. Naphthalene H H C C H C C H C C H C C H C C H H Pyrene H H H C C H C C C C H C C C C H C C C C H C C H H H Note: When the benzene ring is used as a branch, the branch is given the name, phenyl CH3 CH3 H H3C C CH3 CH3 1,2,4-trimethylbenzene 2-phenylpropane Polyaromatic compounds are common carcinogens. They’re also what make grilled meat taste good! 9 FUNCTIONAL GROUPS Groups of atoms in an organic molecule that give it specific chemical and physical properties Alcohols suffix -ol General structure R-OH - R is any carbon chain or ring To name, take name of carbon chain or ring, chop off ‘e’ and add ‘ol’. H C C C O C C O H ethanol Alcohols are commonly used as disinfectants. 2-propanol (isopropyl alcohol) Ethers General Formula R – O – R - R may or may not be different than R To name, put carbon chain prefix together before the word ‘ether’ C C C O C C C O C C methylpropylether diethylether Ethers must be stored in dark containers because they form explosive peroxides over time. Haloalkanes prefixes: fluoro-, chloro-, bromo-, iodo- H Cl C Cl C C C I H dichloromethane 1-iodopropane Chlorofluorocarbons are common refrigerants. Dichloromethane (methylene chloride) is a paint stripper of last resort! 10 Amines General Formula R R N R To name, put carbon chain prefixes together before the word ‘amine’ C C C C N C C C N C C C N propylamine ethylmethylamine C C H N H triethylamine phenylamine Amines generally smell awful. Trimethylamine is responsible for the foul smell of fish. suffix –al O General formula: R C H Aldehydes By definition, double bonded oxygen is at end of carbon chain O O C C C C H C H methanal (formaldehyde) butanal Wine spoils via the conversion of ethanol to ethanal to ethanoic acid (acetic acid, vinegar). suffix –one Ketones O General formula: R C R' By definition, double bonded oxygen is somewhere in the middle of carbon chain. O C C C propanone (acetone) O O C C C C C 2-pentanone (methylpropylketone) cyclohexanone 2-butanone (methylethylketone) is the primary component of nail polish remover. 11 suffix –oic acid Carboxylic Acids O General formula: R C O H O O C C O H C C C C C O H pentanoic acid ethanoic acid (acetic acid) Nylon, the clothing fiber, is the combination of a dicarboxylic acid and a diamine. O H C O C C C O C C C + H C N C C C N C O O C C N C C C N C C C C C C C O 1,6 hexanedioic acid + 1,6 diaminohexane nylon66 Nitro compounds prefix: nitro- General formula: R-NO2 O O O N N O O C C N O nitroethane Many explosives are nitro compounds. N O O 2-methyl-1,3,5-trinitrobenzene (2,4,6-trinitrotoluene) (TNT) The following functional groups only need to be recognized. You will not have to name the compounds 12 Esters O R O C R' H O H H H H H H H H O H H C O C C C C H H C C C C C O C C H H H H H H H H H H H methyl butanoate pentyl ethanoate (pineapple) (banana) General formula: H H O H H H H H H H H H C C O C C C C C C C C C H H H H H H H H H H H ethyl nonanoate (grape) Most esters have pleasant smells and tastes. They are commonly used as artificial flavorings. Amides General formula: O H N C R H O H H 2 2 H N C C C CH3 H O H N C CH3 CH3 butanamide N-methyl ethanamide A protein is a long chain of amide groups linked together. Nitriles General formula: R C N C C N N C C C C C C methanenitrile pentane-2-nitrile Nitriles are very polar; thus, they make good solvents. Paradoxically, nitriles are used to make nitrile gloves which are chemically resistant.