transplants metabolism
advertisement

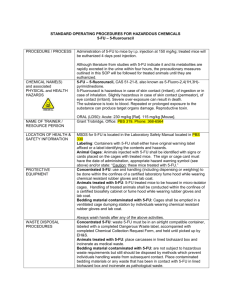
IBC SOP 02 Use of 5-Fluorouracil (5-FU) within the BRC Background: 5-Fluorouracil (5-FU) is a drug used clinically in the treatment of colon cancer. It is classed as a cytotoxic drug (chemotherapeutic). The drug is provided in solution by the pharmacy and then diluted as required. The drug requires metabolism to become active and this only occurs inside of a cell in the body. 5-FU is extensively metabolized (broken down) into inactive components (CO2 and fluorine gas which is breathed out) and a very small fraction of the dose is excreted which is then subject to photodegredation. 5-FU also has a very short half-life in humans and mice and is only active for 30-60 minutes following injection. Application at BRC: 5-FU is used extensively in the analysis of blood cell function in mouse models. The conventional dose used in mouse models is 150mg/kg intraperitoneally. It can be used for several purposes: 1) To haemoablate the mouse through injection of a single dose of 150mg/kg intraperitoneally and allow blood stem cell (HSC) based recovery of the haemopoietic compartment. This assay can be used to monitor the rate or changes in recovery in mutant mouse strains to assist in understanding of the HSC biology in these mice. WT animals would fully recover haemopoietically from this treatment in a period of 4-6 weeks and all would be anticipated to survive a single dose of 5-FU; 2) Repeated doses of 5-FU can be administered (at least 2 weeks apart) as a way to cause HSC stress akin to that of serial bone marrow transplant. This assay can be used to investigate HSC activity after repeated rounds of stress STV IBC SOP 02 Version: 1.0 Approved: 18 March 2010 where defects in self-renewal of the HSCs are more likely to become apparent as a result of the repeated stress. This model also more closely related to the clinical use of chemotherapy where serial doses are administered after a break period. WT animals would recover haemopoietically from this treatment and all would be anticipated to survive; 3) A single injection of 5-FU is administered 4 days prior to bone marrow collection which results in a significant enrichment of the HSC and progenitors as a result of the death of the more mature cells. Handling of Animals: Currently animals treated with 5-FU are considered cytotoxic animals for the remainder of their housing at BRC. This amendment requests a rescheduling of the animals treated with 5-FU after a period of 4 days to being considered non-cytotoxic (Note: this ONLY applies to 5-FU and not other cytotoxic agents). This is because 5-FU has an active time in mice of 30-60 minutes as is near completely metabolized with low toxicity of excreted metabolites. Therefore after several days the animal would be completely free of any 5-FU and there would not be toxic material contained in the cages. Proposed Action: Day 0 Animals administered 5-FU (150mg/kg ip) Cytotoxic label placed on cage and animals handled as cytotoxic and waste handled similarly. Day 4 Animals moved to a new box and considered non-toxic. Previous box waste disposed of as cytotoxic waste and washed and cleaned appropriately according to existing BRC rules. STV IBC SOP 02 Version: 1.0 Approved: 18 March 2010