Section 6
advertisement

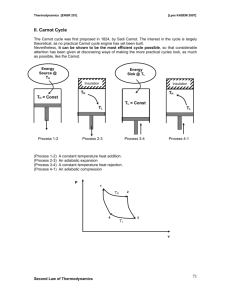
SECTION VI – SECOND LAW OF THERMODYNAMICS Second Law of Thermodynamics: Entropy can be created but not destroyed. Second Law is, unlike the first law, not a conservation law, i.e. entropy, unlike energy, and mass, is not a conserved quantity. Entropy [Greek: (Entrepein; meaning evolution) is a measure of the disorder of the molecules of a system. Entropy can be thought of as a measure of the chaotic nature (the “mixed-upness” or disorder) of a system or state. As a system becomes more disordered, e.g. through the addition of heat, its entropy increases. Second Law: Emphasizes the unidirectional nature of heat transfer and other processes which take place spontaneously; Recognizes that heat and work are not equivalent forms of energy; Establishes formal relationships to augment the first law in thermodynamic analysis Quality of energy of a system: potential ability of a system to do work. Available work or available energy of a system: part of energy of a system that can potentially do work. Esystem = Eavailable + Eunavailable Reversible and Irreversible Processes Causes of irreversibility - heat transfer through a finite temperature difference - mixing or free expansion (in mixing, work must be done to separate the components that are mixed; free expansion is irreversible because of loss of ability to do work) - friction (solid, fluid, drag) (here, work done to overcome friction is lost as useful work) chemical reactions. Reversible processes must be internally (i.e. within the system) and externally (i.e. in the surroundings) reversible (i.e. totally reversible). Reversible processes are ideal ones that preclude dissipative effects. All real processes involve friction from moving parts, heat transfer due to temperature differences, mixing and chemical reactions. Consider the quasi-static (i.e. reversible) expansion of a gas in a piston-cylinder system - Prerequisites: no pressure gradients no turbulence in the fluid Section VI – Second Law of Thermodynamics Page 32 Pext reversible expansion Pgas is infinitesimally larger than Pext Process is infinitely slow Pgas A: Area of piston dx Work done in expansion: dW = Pgas Adx = Pgas d W12 = 12 Pgas d3 W12 = 12 Pgas dv P P1 & are connected by a continuous line since process is reversible P2 v1 v2 v dv 12 Pgasdv is not always work done per unit mass. Consider, e.g., free expansion (i.e. expansion not restrained at a moving boundary) Consider, e.g., free expansion (i.e. expansion ont restrained at a moving boundary) gas (a) P11 Sliding partition evacuated (b) Section VI – Second Law of Thermodynamics Page 33 - when the partition is removed in (a), the gas will expand to a new state (P2,2). The old and new states can be represented on a P- diagram; however, the process can only be shown by a dotted line. Some intermediate states could be found by using a number of partitions as shown in (b) P P1 Intermediate states P2 1 2 gas does no work expanding (no boundary is moved) unless a process is reversible, work done (or work done per unit mass) in expansion or compression will always be less than Pd Consider a simple compressible substance - for a reversible process: W = Pd - Maximum work; no lost work For an irreversible process: W = Wactual + Wlost May be small, large or zero, depending upon the degree of irreversibility Pd = Wlost + Wactual Example: Nitrogen at 500 Kpa and 400K is contained in a closed piston-cylinder assembly that has an initial volume of 750 cm3. The nitrogen is heated isothermally and expands until its pressure is reduced to 100KPa. During this process the work done by the nitrogen amounts to 0.55KJ. Determine whether this process is internally reversible or irreversible. P system boundary P1 N2 T1=400 K P1=500 KPa P2=100 KPa 1=750 cm3 isothermal process Q1 2 W12 = 0.55 KJ P2 1 2 Section VI – Second Law of Thermodynamics Page 34 Can air be treated as an ideal gas under the conditions given? 0.5 0.1 0.029 Pr1 0.147, Pr2 Z1 Z 2 1 3.39 3.39 N2 can be treated as an ideal gas 400 3.175 TR2 126 W12,int.rev 12 Pd Tr1 Ideal gas eqn. of state: P = mRT W12, int rev = 2 1 mRT 2 d d , mRT mRT ln 2 1 1 P11 = mRT1, P22 = mRT2, T1 = T2 P11 = P22 2 P1 1 P2 P 500 W12, int rev = P11 ln 1 500 750 10 6 ln 0.604 KJ 100 P2 W12 actual W12 int rev process is irreversible Statements of the second law Clausius Statement (see p. 213 of text) - governs the operation of refrigerators, air-conditions, and heat pumps Kelvin-Planck Statement (see p. 208 of text) - governs the operation of heat engines, i.e., the steam power plant cycle - Both statements are equivalent expressions of the second law, i.e., a violation of one statement leads to a violation of the other (see pp. 213—214 of text) Perpetual Motion Machine of the second kind - One which will violate the second law i.e., A machine which will produce work continuously while exchanging heat with only a single thermal energy reservoir. Cannot Principles (see p. 224 of text) - An attempt to answer two questions: How much work input is required for the operation of heat pumps and refrigerators? How much heat must be rejected during the operation of a heat engine? - First Proposition: The thermal efficiencies of all reversible heat engines operating between the same two thermal-energy reservoirs are the same. - Second Proposition: The thermal efficiency of a reversible heat engine is greater than that of an irreversible heat engine when both heat engines operate between the same two thermal-energy reservoirs. Section VI – Second Law of Thermodynamics Page 35 The thermodynamic temperature scale and maximum theoretical thermal efficiency th,rev = f (TL,TH) th, rev = Q Wnet Q H Q L 1 L f T2, TH QH QH QH Q L QH gTL, TH rev Consider an intermediate temperature (THL) thermal-energy reservoir QL QH TH > TL Q HL gTHL, TH QH QH Engine A gTL, TH WA net QL gTL , TH Q HL QHL QL QH THL QHL Engine B QL TL Q L Q HL Q HL Q H gTL, TH gTL, THL gTHL TH WBn et L.H.S. is independent of THL R.H.S. must also be independent of THL form of the function must be such that TL gTL, THL THL (THL ) g (THL,TH) = (TH ) Since (THL) will cancel from the product g (T2,THL) g (THL,TH) Q L QH (TL ) - this can be satisfied by a number of temperature functions (TH ) (T ) T (T ) : temperature function called “absolute temperature” by Lord Kelvin Section VI – Second Law of Thermodynamics QL QH threv = 1 T L TH th,rev (carnot ) = 1- Page 36 TL TH (carnot) Tlow T 1 low 0 Thigh Thigh Tlow > 0 zero zero absolute temperature thermal-energy reservoir is not attainable (since heat transfer is then impossible) 3rd Law of Thermodynamics Consider a carnot heat engine (i.e. reversible heat engine). Let the engine receive heat at the temperature of the steampoint and reject heat at the temperature of the icepoint. th engine 1 Ticepoint TL 1 TH Tsteampo int If th engine were measured, it will be 26.8 % Ticepo int Ticepo int 1 0.268 0.732(i) Tsteampo int Tsteampo int Tsteampoint – Ticepoint = 100 (ii) Solving (i) & (ii) simultaneously, Tsteampoint = 373.15 K Ticepoint = 273.15 K Coefficient of Performance of a Carnot Refrigerator Q evap (COP)R = - Q evap Q cond (COP) R carnot 1 Q cond 1 Q evap 1 TH 1 TL Coefficient of Performance of a Carnot Heat Pump (COP) HP = Q cond Q evap Q cond 1 Q evap 1 Q cond COPHP Carnot 1 T 1 L TH Section VI – Second Law of Thermodynamics Page 37 The work output from a Carnot engine is used to drive a Carnot refrigerator. The engine receives 75KJ of heat from a thermal-energy reservoir whose temperature is 600°C, and it rejects heat to the surrounding air, which is at 30°C. The refrigerator is to be used to maintain a refrigerated space at -25°C. The refrigerator also rejects heat to the surrounding air. Determine the cooling load that the refrigerator is capable of handling. QL=Qe Refrigerated space TLK 25C Carnot Refrigerato r QH R wnet TH L 600C QH E 75KJ Carnot Engine: th = 1 Carnot Heat Engine TL 273 30 0.653 TH 273 600 Wnet = th Q H E = (0.653)(75) = 48.98 KJ Carnot Refrigerator: (COP)R = 1 THR 1 TLR (COP)R = 4.509 (COP)R = - QLR W net QLR COPR Wnet QLR 4.509 48.98 220.9 KJ 1 (273 30) 1 (273 25) Surroundings air T H 30C R = T LE