A thin
advertisement
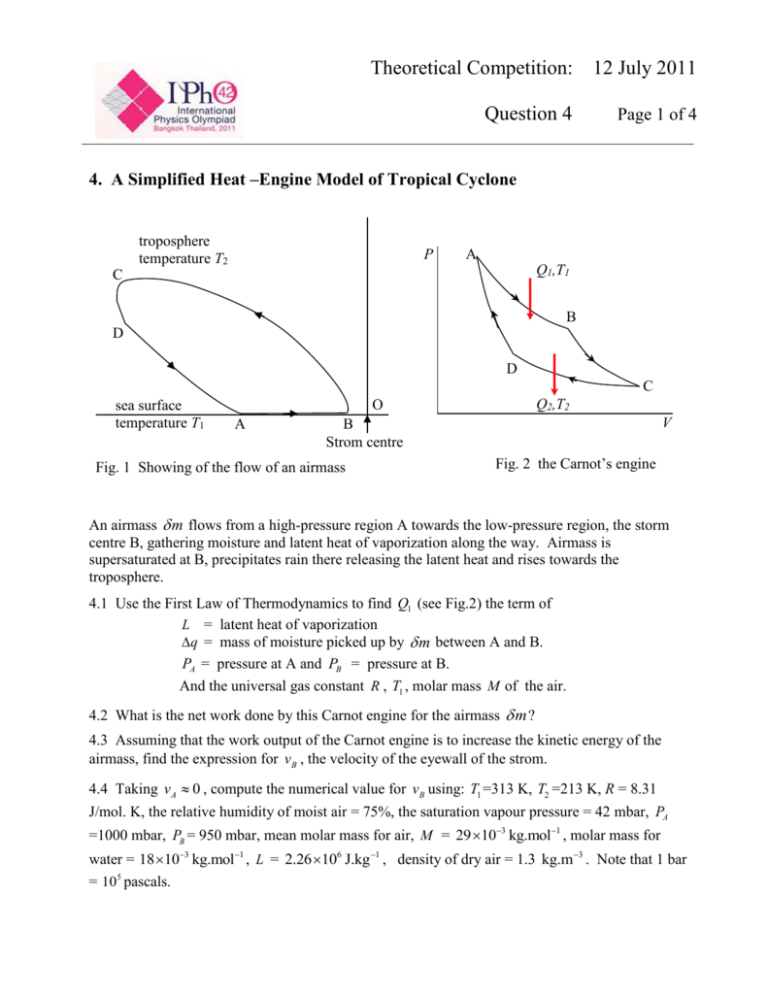
Theoretical Competition: 12 July 2011 Question 4 Page 1 of 4 4. A Simplified Heat –Engine Model of Tropical Cyclone troposphere temperature T2 P A Q1,T1 C B D D C sea surface temperature T1 Q2,T2 O A V B Strom centre Fig. 1 Showing of the flow of an airmass Fig. 2 the Carnot’s engine An airmass m flows from a high-pressure region A towards the low-pressure region, the storm centre B, gathering moisture and latent heat of vaporization along the way. Airmass is supersaturated at B, precipitates rain there releasing the latent heat and rises towards the troposphere. 4.1 Use the First Law of Thermodynamics to find Q1 (see Fig.2) the term of L = latent heat of vaporization q = mass of moisture picked up by m between A and B. PA = pressure at A and PB = pressure at B. And the universal gas constant R , T1 , molar mass M of the air. 4.2 What is the net work done by this Carnot engine for the airmass m ? 4.3 Assuming that the work output of the Carnot engine is to increase the kinetic energy of the airmass, find the expression for vB , the velocity of the eyewall of the strom. 4.4 Taking vA 0 , compute the numerical value for vB using: T1 =313 K, T2 =213 K, R = 8.31 J/mol. K, the relative humidity of moist air = 75%, the saturation vapour pressure = 42 mbar, PA =1000 mbar, PB = 950 mbar, mean molar mass for air, M = 29 103 kg.mol1 , molar mass for water = 18 103 kg.mol1 , L = 2.26 106 J.kg 1 , density of dry air = 1.3 kg.m 3 . Note that 1 bar = 105 pascals. Theoretical Competition: 12 July 2011 Question 4 Page 2 of 4 4.5 Given that the azimutual velocity v and the radial distance r from the eyewall of the cyclone are 1 such that vr 2 constant, and that the velocity v A at the outer edge of the cyclone is 10 m/s, calculate the effective radius of the cyclone. [Take rB to be 10 km.] Theoretical Competition: 12 July 2011 Question 4 Page 3 of 4 SOLUTION Q1 U P V 4.1 [The first law of thermodynamics] …………….. (1) Where U CP T L q, T T1 constant T 0, P V V P Hence Q1 L Q V P Q1 L q Q1 Lq Q1 Lq m PV Note also that M mRT1 M M PB P 1 P PdP, PA ln …………….. (3) RT1 m RT1 mRT1 M …………….. (2) q dq from A to B PA PB …………….. (4) 4.2 Net work done by the Carnot engine is W Q1 Q2 …………….. (5) And according to the Second Law of Thermodynamics, we have 4.3 Q2 Q 1 T2 T1 …………….. (6) T W 1 2 Q1 T1 …………….. (7) T mRT1 PA W 1 2 Lq ln M PB T1 ………….. (8) T mRT1 PA 1 ln m vB2 vA2 1 2 Lq 2 M PB T1 1 vB T q RT1 PA 2 2 2 1 2 L ln v A PB T1 m M Theoretical Competition: 12 July 2011 Question 4 Page 4 of 4 T2 303 213 0.297 1 = 303 T1 RT1 PA ln 4454 J.kg 1 M PB 4.4 q 100% 75% of mass at saturation vapour pressure 0.0075 kg.m3 q 0.0058 (dimensionless) m q L 13108 J.kg 1 m vA 0 vB 102 m/s = 368 km/h 1 1 1 4.5 From vr 2 = constant, we have vA rA2 vB rB2 , rA 1040 km











