The organism E - BioMed Central
advertisement

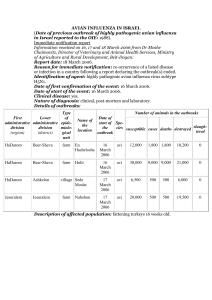
### Detailed response to comments by Professor Tauxe (his comments in italics) The organism E. coli O157:H7 survives in non-human reservoirs, where it is sustained by continuous transmission, and it episodically overflows into the human population, where it is not sustained. Since we have known since the 1980's that E. coli O157:H7 has caused a number of outbreaks in day-care settings characterized by child-to-child transmission, the conclusion that person-to-person spread is significantly associated with age is not news. We would agree that there have been previous reports of the association of person-to-person spread with age. However, most of these were within the framework of individual outbreaks or limited to outbreaks within a single household. We believe that our paper provides the first standardised analyses of the relationship between primary mode of transmission, mode of secondary transmission, and age (as well as country) in outbreaks from differing surveillance and reporting systems. The fact that our analyses help confirm the findings of other studies we take as a strength of our study as opposed to a weakness. In this regard we have modified our text in the discussion. The conclusion that the likelihood of secondary transmission varies by primary mode of transmission appears to be the direct result of the definitions you used, since you define several of the primary modes as “secondary transmission”. We do not completely agree here as we would argue that our results are not purely a function of our choice of definitions. We have defined a secondary case (we did not originally give an explicit definition of the term "secondary transmission") as a case "with no reported exposure to the original source of contamination", while a primary case did have reported exposure to the original source of contamination. Each outbreak was then, by our definitions, categorized by mode of primary transmission AND mode of secondary transmission based on the putative source of primary contamination and the predominant putative source of secondary spread. Therefore, our categories are different from those used in many surveillance systems or published papers, because outbreaks are defined by the primary, initial source of infection and the most frequent mode of secondary transmission, rather than solely by the numerically predominant type of transmission for all the outbreak cases as a group. For example, if an outbreak involving two primary cases who had direct contact with animal faeces, but 18 cases who acquired infection by person-to-person spread, was categorized by the original reporters as being a “person to person” outbreak, we would define this incident as having animal contact as the primary mode of outbreak transmission, and person to person spread as the mode of secondary transmission. We created definitions based on the aims of our study and the methods that embodied these, 1) because we used information from case reports, and did not have the original surveillance data with mode of transmission classified, 2) because for some outbreaks there was a lot of uncertainty in source attribution, and 3) because our study included outbreaks from differing surveillance and reporting systems. Therefore we felt it was important to consistently apply standardised definitions of mode of primary/secondary transmission based on all the data provided in the report, and not to use definitions from any specific country. 106734093 1 Additionally, we felt that, epidemiologically and in terms of outbreaks, the main difference (for the purposes of this study) is between those persons who acquire infection from the initial source, and those who get it from another person or from a body of water, food or other item contaminated by another person. For the former, transmission can only be stopped by preventing contact between the humans and the original source (eg animals/contaminated water) or by preventing contamination of the initial source. If there are no secondary cases, we would argue an outbreak is highly unlikely to spread infinitely since either the initial source would either naturally be cleared of bacteria or an outbreak investigation would identify the source and/or effect preventive measures. Thus, the cases which we defined as secondary acquired infection by person to person spread, with a few involving water as a mode of secondary transmission between two persons. Therefore, some, but not all, waterborne cases were defined as secondary in our study. We made this distinction because information provided in outbreak reports appeared to indicate that some waterborne cases were most likely to have been caused by water contaminated by another human (i.e. in a pool, or in a beach/swimming hole where there is not an obvious natural reservoir for the bacteria), whilst in other outbreaks there was evidence of non-human faecal contamination of the water. We would suggest that this is a significant difference, primarily because prevention of such outbreaks is different. The steps needed to reduce the likelihood of human contamination involve creation and enforcement of policies to prevent human faecal contamination (i.e. policies that enforce exclusion of non-toilet trained children from pools, immediate closure of contaminated pools, exclusion of persons who repeatedly violate or ignore rules etc.). On the other hand, preventing wildlife contamination of water sources is quite different. In particular, water sources where animals have access – i.e. lakes, rivers and swimming holes - generally cannot be chlorinated, and exclusion of humans is easier than exclusion of animals. The text of the paper has been edited to clarify the reasons for creating the new definitions. Additionally, the definition section has been clarified to make it clear that outbreaks were defined by both primary and secondary modes of transmission. Given those definitions, the outcome seems to be fore-ordained. The fact that you categorized all outbreaks with no identified source or exposure to risk factors as “secondary transmission” is unusual to say the least, and makes your conclusions difficult to interpret. Again, we cannot completely agree with the referee here. We would absolutely agree that our definitions are not ideal, but given the variations in definitions between countries, within countries and over time, a standard definition needed to be selected to allow comparison As stated above, each outbreak was classified both by modes of both primary and secondary transmission. (The definitions we used have now been clarified in the paper text as stated above). If the primary initial source was not clear from the information provided, the outbreak was defined as primary source unknown, irrespective of whether or not secondary transmission occurred. In fact almost 30% of the outbreaks had an unknown mode of primary transmission, which would not be unusual, given the difficulty of identifying initial source in some situations. The phrase "no identified source or exposure to risk factors" refers to the definition of secondary 106734093 2 cases, not secondary outbreaks. Changes, mentioned above, were made to clarify the difference in classification of modes of primary/secondary transmission and primary/secondary cases. Applying these case and outbreak definitions may have resulted in some degree of difficulties of classification, depending on the detail of information available from the outbreak paper/report, but as mentioned above, standard definitions were needed in order to allow for comparison of data from multiple reports and countries. Not surprisingly however, since our data set only included outbreaks that were investigated and were either unusual or large enough to be written up in a report, at least some information was available in regards to the mode of secondary transmission. For instance, at least one included outbreak was picked up by health authorities when infection was diagnosed in a group of children, all of whom had been swimming or had siblings who went swimming at a particular swimming area. The available evidence suggests that the pool was contaminated by one or a couple of persons, but by the time the outbreak was recognized, the identity of that person(s) and initial primary source of infection (contaminated food, touching a cow etc.) could not be determined. Thus while we do not know the primary mode of transmission, the mode of secondary transmission is known, and so the outbreak can be included in analyses of mode of secondary transmission. We felt that by including outbreaks where the primary mode of transmission was not known as a separate category, we might be able to learn something about the composition of these outbreaks based on the similarities and differences to other categories. In fact, we repeated analyses excluding outbreaks with an unknown mode of primary transmission, to determine what affect those outbreaks might be having on the outbreak. The discussion has been expanded to include mention of the reasons for including outbreaks with an unknown mode of transmission and our findings involving these outbreaks. Rangel (your reference 32), reports that 14% of outbreaks were the result of person to person transmission, and another 21% were of unknown route. Using outbreak associated cases as the index, then p-p was 8% of cases and unknown route was 9% of cases, a sum of 17%, close to your estimate of 20%. But there is no clear reason to do that with the outbreaks of unknown route. We have to admit to not quite understanding the point that is being made here. However, we think it may be that we need to give greater clarification as to how modes of primary and secondary transmission are defined. We categorise outbreaks by firstly the specified or most likely primary mode of transmission, which is inherently defined as one which does not involve passage via or through another human. If applicable, we then additionally categorised each outbreak by the mode of secondary transmission, The outbreak (as opposed to case) categories provided by Rangel and which are used in some surveillance systems tend to be what could be described as the main or predominant mode of transmission – i.e. the mode that accounted for the majority of all the detected cases. As in the example above, this did not necessarily identify or reflect the primary or initial source or mode of transmission - i.e. one child might have become ill after eating contaminated beef, but if the infection spreads from this child, to others in a nursery, the numeric majority of cases would be the result of person to person spread. We believe we have clarified this distinction and the definitions in the Methods and Materials and Discussion 106734093 3 With specific regard to person to person outbreaks, although these may account for 8% of cases, but each of those outbreaks involved cases which were primary as per our definitions, and others which were due to person to person spread and were therefore, using our definitions, secondary cases. We felt we must exercise caution about categorising outbreaks where the mode of primary spread was not clear – we may be able to identify the mode of secondary transmission for the outbreak (eg via water, via family contact), but know little about the initial primary source (eg whether the individual/s who introduced infection into a pool or household acquired it from direct animal contact, or from contaminated food etc.). By creating a category of unknown primary mode of tranmission, we tried to use the data to provide some informed indications as to what might have happened in these outbreaks. We accept that this was not clear enough in the original manuscript and have hopefully now clarified this in the modified text Establishing the modes by which transmission occurs is important, to guide public health action and prevention strategy. It has long been established that limited direct person-to-person transmission occurs in particular settings. The core modes of transmission for E. coli O157 are through food, water, direct contact with animals or environment (other than food or water), and person-to-person. Each of those implies a different set of protective strategies. Waterborne outbreaks are then usually broken down into those related to water for drinking and those related to water for recreation, again because that relates to how transmission is prevented. It is actually unusual for the “original” source of the contamination in food or water to be established with certainty, though we assume that for beef it is the cow, and for swimming pools it is someone in the pool themselves. This is much less clear for a food like alfalfa sprouts, where the seeds were perhaps contaminated a continent away and years ago - who knows what the source of that contamination was? Similarly, when a body of natural water is contaminated with both human or animal feces, it may be difficult to decide whose was the source. Even if it appears to be personto-person from a human source, it is usually impossible to be sure who was the first case, and from where they got their infection. I therefore do not find the term “indirect person-to-person transmission” useful as a general descriptor, and I suggest you abandon it. You can simply label some waterborne outbreaks as being related to human fecal contamination or foodborne outbreaks related to an infected foodhandler. We are a little confused by comment here as we do not believe we have used the term “indirect person-to-person transmission” at any point in the manuscript The only time 'indirect' is used in the introduction: "secondary transmission from an infected person, directly or indirectly[18] to another person, also occurs…" However, we would agree that it is often very difficult to definitively establish the initial source of infection/contamination. That is the reason why we would refer to these as 'suspected' modes of initial/primary transmission, and why we kept an unknown category. But we believe that in order to try to derive some meaningful learning from analysis of past outbreaks, you have to categorise things somehow – our categories for mode of initial/primary transmission are generally equivalent to the categories used by the surveillance agencies (with minor modifications, the primary categories are almost exactly as used by the US/CAN/UK). Finally, we believe that although there are merits to the referee's suggested framework, it would have meant excluding further outbreaks from our analyses because we needed to compare general 106734093 4 categories and dividing the data into very small, albeit precise proportions, would have created problems in terms of category size. We believe that the implications for prevention and control of the different types of mode of secondary transmission were sufficiently common within each category to justify not subdividing them further. The discussion was expanded to include a couple of sentences explaining the decisions to stick with relatively broad categories for primary and secondary transmission. From a public health point of view, it would be useful to categorize the outbreak series into those four basic modes, and then summarize for each mode the % of cases that are related to a different mode or site of transmission than the one that predominates in an outbreak (which is my understanding of the rather vague term “secondary transmission”) as well as the % that were related to direct person-to- person transmission in each mode. For example, a major foodborne outbreak might be followed by small swimming outbreak caused by the same strain. Those would be secondary cases, not because they are “indirect person-to-person”, but because they did NOT consume the contaminated food, but had a different exposure in common. As mentioned above, we did not use the term "indirect person-to-person transmission " in our paper in the form that it is quoted by the referee. We attempted to give a very clear definition for a secondary case as opposed to mode of secondary transmission. We also distinguished between initial/primary mode of outbreak transmission, and mode of secondary transmission, and between primary and secondary cases. We have though re-examined the text and have hopefully made this distinction very clear. Finally we are completely in agreement with the last sentence, at stated in our definitions section"…no reported exposure to the original source of contamination…". It is worthwhile drawing attention to the observation that there can be more than one mode of transmission in one outbreak. We agree with the referee here – that was also our reason for distinguishing between primary and secondary modes of transmission. In order to make the statistical analysis possible, we had to define a single primary and single (where applicable) mode of secondary transmission for each, but we do acknowledge specifically in the methods and materials that some outbreaks had multiple methods of both primary and secondary transmission (and explaining the process used to select the mode of secondary transmission when the reported suggested multiple modes). In addition, there are often some person-to-person cases in outbreaks where the initial source was contaminated food, water or animals . This emphasizes the need to educate all cases about handwashing, etc. in the outbreak setting. The reviewer’s comments suggest that those are separate, though related, observations. We agree with the referee here, and we had hoped that we had raised this very point with the very last sentence of the paper: " This study does, however, emphasise the importance of simple but effective strategies, such as handwashing, which can reduce the risk of person to person transmission, particularly amongst young children." We have however, now also mentioned this earlier in the discussion It is not particularly surprising that there are few differences across countries in the proportion due to secondary transmission. This is related to basic biology of the infection, the infectious dose, the degree of shedding, etc., as these are factors that are not likely to vary widely across 106734093 5 different industrialized nations. I encourage the authors to read a study of shigellosis spread in households as a function of age of the first case. Wilson R, Feldman RA, Davis J, LaVenture M. Family illness associated with Shigella infection: the interrelationship of age of the index patient and the age of household members in acquisition of illness. J Infect Dis. 1981 Jan;143(1):130– 132. We would quite strongly argue on a number of issues here – firstly we are not aware of any published, standardised statistical comparison has been carried out involving multi-country data, and therefore we believe it is of interest and importance to look at the potential variation between countries. Although we agree with the referee's comments on the factors which suggest there would be no difference, we did not wish to assume this when we had the opportunity to test it, even bearing in mind the limitations of available data. In addition, we would suggest that the biology and shedding are not necessarily entirely the same between countries: the distributions of phage types and shedding are different across countries, even those sharing large land borders (such as Scotland compared to England). In addition, we assume that most Scottish cases ultimately acquired infection from British (or probably Scottish) cows, whereas the Canadian cases, for instance, are from Canadian cows (or US cows via California spinach…). It is entirely possible that there are strain differences between countries, and those strain differences could affect the way the bacteria affects humans (bearing in mind the evidence that different strains possess different virulence factors/combinations, of which some are more strongly associated with severe disease/HUS than others). We did not overdwell on this in the original manuscript as we were concerned about straying too far from the scope of the paper. However, we have now added to the discussion regarding what might be expected and what was observed with respect to variation between countries. ### Detailed response to comments by Mohamed A Karmali (his comments in italics) This is a well designed systematic review, probably the first one on this topic, of published reports of E.coli O157 outbreaks from several countries, aimed at characterizing the proportion of secondary cases and analyzing the relationship of the latter to the primary cases. Methodology and analyses are sound. The study, in general, corroborates, but with greater precision, the findings of several earlier descriptive studies of individual outbreaks. In particular it shows that secondary transmission is common, and that it is significantly influenced by age and modes of primary and secondary transmission, but not country. The finding that secondary transmission is highest in infants and young children leads the authors to suggest that "prevention should focus on reducing spread amongst young children, especially in locations they frequent, such as nurseries", a suggestion that is not novel given that the E. coli O157:H7 infection has long been known to have its highest age-specific incidence, and complications such as hemolytic uremic syndrome in this age-group. We agree with the referee's above comments, and have clarified the quoted section in the abstract and related section in the conclusion to confirm that our suggestion on prevention emphasis is not 106734093 6 novel and that we see our results as re-affirming present efforts to reduce transmission amongst young children. The influence of the possible lack of immunity in explaining the occurrence of secondary cases in young children should be discussed. Many infectious diseases - mumps, measles, rubella, chicken pox, salmonellosis, campylobacteriosis - to name a few, have their highest age-specific incidence in the young. Secondary transmission may be a major factor in this although the latter probably occurs because of a lack of immunity in naive individuals who are exposed to a pathogen for the first time. Lack of immunity is likely also to be one of the key expnantions for a high incidence of disease whether primary or acquired by secondary transmission, in the young. We acknowledge that we implied the role of immature immune systems in the young, rather than being explicit about it. The paper now includes comment on this. While we agree with the referee that it would be of interest to discuss other aspects of the role of immunity, we are little concerned that any detailed discussion of this is outwith the scope of the paper. There is some evidence that farming families or workers who are exposed long term have some immunity to pathogenic E. coli[1,2]. We have therefore added a couple of short sentences to the discussion to mention the potential role of immunity, while trying to not discuss immunity in too much detail. . Reference List 1. Wilson JB, Spika J, Clarke RC, McEwen SA, Johnson R, Rahn K, Renwick S, Karmali M, Lior H, Alves D, Gyles CL, Sandhu K: Verocytotoxigenic Escherichia coli infection in dairy farm families. Journal of Infectious Disease 1996, 174: 1021-1027. 2. Silvestro L, Caputo M, Blancato S, DeCastelli L, Fioravanti A, Tozzoli R, Morabito S, Caprioli A: Asymptomatic carriage of verocytotoxin-producing Escherichia coli O157 in farm workers in Northern Italy. Epidemiology and Infection 2004, 132: 915-919. 106734093 7