Zhouravleva
advertisement

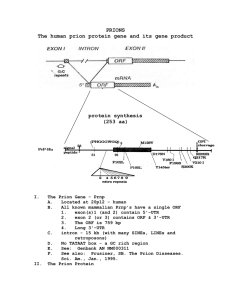
THE ROLE OF PRION DOMAINS IN EVOLUTION Galina A. Zhouravleva Department of Genetics, St. Petersburg State University, St. Petersburg, Russia Prion (from proteinaceous infectious particles) is a protein isoform that is able to convert the normal form of the same protein into prion form. This term was proposed by S. Prusiner in 1982 to explain the unusual properties of pathogen transmission observed in the cases of some neurodegenerative diseases in mammals. Examples of the “prion diseases” include “mad cow disease”, or bovine spongiform encephalopathy (BSE), sheep scrapie disease, and human Creutzfeldt-Jacob disease. Importance of prion diseases was emphasized by the possibility of BSE transmission from cows to humans. Prion capability is not restricted to PrP and can be found in the other proteins. Moreover, discovery of the yeast prions [PSI+], [URE3] and others makes it clear that PrP represents just an extreme case of much more common phenomenon. Experiments using yeast and fungal systems confirm that prion-like phenomena are widespread, and identify the major components of the cellular machinery that modulates prion formation and propagation. The prion concept reveals a new mechanism of inheritance, which operates at the level of the structural organization of proteins. Possibly, the prion formation is a pathological process, while conservation of prion-forming potential in evolution is due to some adaptive functions played by PFDs (prion forming domains) in certain conditions. As misfolded and potentially aggregating proteins are usually accumulated during aging, it is an intriguing possibility that aging could promote prion-like pathologies. Indeed, some aggregation-related diseases (e.g., Alzheimer's disease) in humans are frequently associated with advanced age. An alternative model suggests that prion formation by itself could be an adaptive process, so that certain prions are responsible for the emergence of the adaptive traits. Various models, explaining the biological roles and evolutionary conservation of prion-forming processes, and relating them to the certain controversial aspects of the theory of evolution are discussed. This work was supported by the Russian Foundation for Basic Research (03-0448886) and by the Presidium of RAN (Program “Biosphere origin and evolution”).