1 Louisa Ulrich-Verderber University of Chicago Biotechnology 07
advertisement

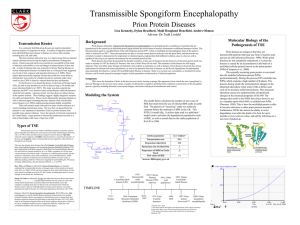
1 Louisa Ulrich-Verderber University of Chicago Biotechnology 07/18/13 Prions: An UN-folding Mystery Disease has always been at the forefront of concerns to medical professionals and scientist, especially when these diseases come from other animals. This is partially the case in Prion Disease, a neurological condition that has the ability to jump between species. There is no way to know how long Prion Disease has been inflicting human populations as the molecular structure and mechanism are complex. In order to attempt to treat and prevent this disease it is important to first understand the disease itself. A common form of Prion disease, Scrapie, was first reported in England in 1732 and Stanley B. Prusiner discovered prions in 1982 and was awarded the Nobel Prize for his work in 1997. ("Prion Disease.", 1) The first recorded major outbreak of was in central New Guinea in 1950. The native people called the sickness “Kuru” or “trembling in fear”. The infected patients lost the ability to walk or swallow, and later died of malnourishment. In 1960 experiments on chimps conclusively showed that this illness was contagious, and in the 1980’s 145 people died due to infected medical treatments and procedures. The most recent wave of prion related incidents was in 2005 when the beef of infected cows was found to be fatal to humans, and as a result 115 people died in Brittan. Unfortunately these diseases have a long incubation period and lay dormant for years before surfacing again. ("PRIONS:”, 1) 2 Prions are proteins, specifically PrP proteins, that possess the unique ability to reproduce on their own, but can become infectious and potentially very harmful. The harmless variation, PrP-sen is found in all normal human bodies. PrP-sen is located in the brain and is present in neurons. Though the exact function of this protein is unknown, it is thought to be connected with intercellular communication, metal ion transport, and blood cell manufacture. ("PRIONS:”, 1) The harmful variation of PrP, PrP-ren, is a self-propagating miss folded protein that is responsible for a number of diseases that affect the brain and other neural tissue. ("Prion Disease.", 1) Organisms with this form of PrP develop a spongy tissue structure. Prions, like viruses, contain no genetic material within the cell itself, yet once and organism is infected the populations of infectious prions increases. This is due to PrP-ren’s ability to cause other proteins to miss-fold, creating more PrP-ren within an organism. When 3 enough PrP-ren accumulates they form amyloid fibers, which are toxic to cells, causing holes to develop in brain tissue. ("PRIONS:”, 1) The actions of PrP-ren are known as Prion Disease and impair brain function, causing memory changes, personality changes, and a decline in intellectual function. Patients with this disease succumb relatively quickly, dying within a few months to several years. Fortunately, for the general population this disease is very rare, effecting about one person per million worldwide each year. Other names for Prion Disease include: Inherited Human Transmissible Spongiform Encephalopathies, Transmissible Dementias, Transmissible Spongiform Encephalopathies, and TSEs. Prion disease can also infect animals; ingesting meat from infected animals is a major way pathway of transmission of the disease as seen above. ("Prion Disease.", 1) The most well known form of prion disease in animals is Mad Cow Disease, also known as BSE (bovine spongiform encephalopathy). This too is a neurological disorder 4 with an incubation period of about four years. It too causes spongy degeneration within the brain and spinal cord. ("About BSE.", 1) Along with BSE, Scrapie is also well known in agriculture. This is much like BSE only it affects sheep and goat. Unlike BSE animals are reported to insistently rub their fleeces against trees or rock to quench the itching that accompanies the disease. Other symptoms of Scrapie include altered gaits, and convulsive collapse. (Hunter "Scrapie”, 1) When elk and deer acquire a form of Prion Disease it is called Chronic Wasting Disease, which also effects the nervous system. ("Chronic”, 1) We are only recently discovering how all these diseases are transmitted. There are several modes of infection. The first, and most prominent, is the ingestion of infected tissues. This was the case of the 2005 outbreak when cattle were infected with BSE by eating feed that contained the ground up brain material from other cows. Then their meat was made into hamburger, which was later ingested by oblivious consumers who later contracted the infection and died. ("PRIONS:”, 1) Prion Disease can also be transmitted through shared livestock feed. Researchers found that prions also reside in taste buds and tongue tissue of cattle and sheep, which if infected, can cause miss folded PrP-rens to be spread through communal food troughs, infecting whole herds of livestock. ("Chronic”, 1) Prion Disease is also genetic. Mutations in PRNR gene that code for the harmless PrPsen protein can cause PrP-ren to be produced, and lie dormant for years without surfacing in an individual. If this individual were to reproduce before symptoms arose then their offspring could also be infected. ("Prion Disease.", 1) Unfortunately, due to their structure prion proteins are impossible to denature. In fact, they are partially immortal because even heat does not denature them. Their disposal and 5 decontamination of the disease is also very difficult. Fortunately a vaccine has been developed in mice with potentially positive implications for humans. However, due to their long incubations period and highly unpredictable modes of transition it is nearly impossible to track and attempt to eradicate Prion Disease. ("Prion Disease.", 1) Prion Diseases are highly infectious neurological diseases that are crossing species’ barriers from livestock to humans. These diseases can lie dormant for extended periods and kill quickly once they become active. Research into treatment and prevention is currently on the rise, but the future still seems bleak. \ Work Cited: "About BSE." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 21 Feb. 2013. Web. 09 July 2013. "Chronic Wasting Disease." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 4 Sept. 2012. Web. 09 July 2013. Dance, Amber. "Prions Jump Species Barrier." Nature.com. Nature Publishing Group, 04 Sept. 2008. Web. 09 July 2013. Hunter, Nora. "Scrapie—Uncertainties, Biology and Molecular Approaches." ScrapieUncertainties, Biology and Molecular Approaches. Science Direct, June 2007. Web. 10 July 2013. The Johns Hopkins University. "Prion Diseases." Johns Hopkins Medicine, Based in Baltimore, Maryland. The Johns Hopkins University, n.d. Web. 09 July 2013. "Prion Disease." - Genetics Home Reference. Genetics Home Reference, May 2007. Web. 09 July 2013. "Prion Diseases." Johns Hopkins Medicine, Based in Baltimore, Maryland. The Johns Hopkins University, 2013. Web. 09 July 2013. "PRIONS: ON THE TRAIL OF KILLER PROTEINS." Prions: On the Trail of Killer Proteins. University of Utah, 2013. Web. 09 July 2013. "Viroids and Prions." Boundless. N.p., n.d. Web. 09 July 2011 6