Production, Purification, and Cleavage of Tandem Repeats of
advertisement

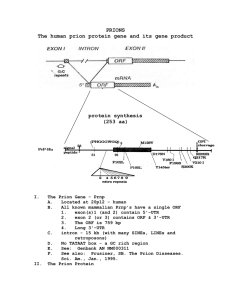
Production, Purification, and Cleavage of Tandem Repeats of Mouse Prion Peptide 106-126 Adviser: Cheng-I Lee Speaker: Shuei-Ling Chang Date: 20120420 Prion diseases are fatal neurodegenerative disorders that can be infectious, genetic, or sporadic in animals and humans. These diseases include scrapie in sheep, bovine spongiform encephalopathy (BSE) in cattle, and sporadic and variant Creutzfeld-Jakob disease (CJD) in humans. These diseases are due to conversion of prion protein from the normal cellular isoform (PrPC) to pathogenic isoform (PrPSc). PrPC is soluble, protease-sensitive, and has high α-helical content, whereas PrPSc is insoluble, protease-resistant, and aggregated with high β-sheet content. Research on the toxicity of PrPSc can help us to understand the mechanism of prion diseases, and to find the therapy for these diseases. The cellular prion protein consists of 208 amino acids (residues 23-231). A synthetic peptide 106-126 of PrP (PrP106-126) has been used as a model to study the toxic properties of PrPSc because this peptide is highly fibrillogenic and toxic to cultured neurons. It is believed that PrP106-126 interacts with neuronal PrPC and subsequently converting PrPC into toxic PrPSc. In this study, we used template repeated polymerase chain reaction (TR-PCR) to develop a tandem repeats of mouse PrP106-126 interspersed by single methionine residues that can be cleaved by cyanogen bromide (CNBr) into homogeneous peptide units. Our preliminary results show various repeats of PrP106-126, indicating that this TR-PCR experiment for construct the tandem repeat PrP106-126 is feasible. However, there are some mutation and deletion in the sequence even though we used proofreading Pfu DNA polymerase in TR-PCR experiment. We will try more conditions to improve the accuracy of the target sequence. Then, we will subclone this DNA fragment into an expression vector to produce fusion proteins in E. coli. The expressed tandem repeat PrP106-126 will be purified with Ni2+-column and cleaved with CNBr into multiple moPrP106-126 units. Subsequently, the toxic effect of recombinant moPrP106-126 in neuron cells will be investigated. We expect the recombinant PrP106-126 that produced by E. coli can replace synthetic PrP106-126. Furthermore, this experiment design provides a new expression system to produce short peptides.