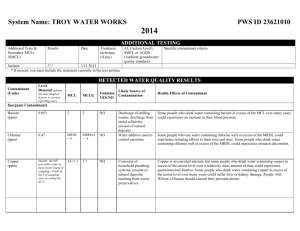
Water Quality Index Scale
advertisement

Water Quality Index Scale 91-100: Excellent water quality 71-90: Good water quality 51-70: Medium or average water quality 26-50: Fair water quality 0-25: Poor water quality Fecal Coliform Bacteria Test Fecal Coliform Bacteria are naturally present in the human digestive track. This bacteria should be rare or absent in “unpolluted” waters. Coliform bateria should not be found in sources of drinking water. Their presence in water serves as a reliable indication of sewage or fecal contamination. Less than 1000 bacteria colonies per 100 mL of water is acceptable by the government for waters used for swimming purposes, while less than 5000 BC/100mL are allowed for waters used for boating or fishing. This test will indicate if your sample has above or below 200 BC/100mL, thus indicating if fecal matter has contaminated your sample. Directions: 1. Since this test requires a 48-hour incubation period, all the water samples have already been tested earlier this week. 2. Find the vial labeled with the same letter as your beaker. 3. Compare the color of the fluid inside the beaker to the Coliform color chart. 4. If your result is Negative your Qvalue is 100; if positive, your Q-value is 65. Dissolved Oxygen Test This test measures the amount of oxygen dissolved in the water. Dissolved oxygen (DO) is important to the health of aquatic ecosystems because all aquatic organisms need oxygen to survive. The DO in water is available to fish, invertebrates, and aquatic plants. If the levels are low, then the organisms might drown. Low levels of DO in water are signs of possible pollution. The percent saturation of dissolved oxygen in water is an important measurement of water quality. Cold water can hold more DO than warm water; therefore, the temperature of the water must be accounted for. Directions: 1. Record the temperature of your water sample. 2. Fill the small tube all the way to the top with water from your sample. 3. Drop 2 DO test tablets into the small tube. It’s ok if some of the water overflows. If water does not overflow, you do not have enough water in your tube! 4. Screw the cap on the tube. More water will overflow as the cap is tightened. Make sure that there are no air bubbles in the sample. 5. Mix/shake the tube until the tablets disintegrate. 6. Wait 5 minutes for the color to develop. 7. Compare the color of your sample to the DO color chart. This will give you the ppm DO in your sample. 8. Look at the % Saturation Chart. Use the temperature and the ppm DO of your sample to determine its % saturation. 9. Now, determine and record the Q-value using the 2nd Chart provided. Nitrate Test Nitrate is a nutrient needed by all aquatic plants and animals to build protein. It is a measure of the oxidized form of nitrogen. The decomposition of plants and animals, as well as the excretions from living animals can release nitrates into the aquatic system. An excess of this nutrient can plant grow and decay and promote bacterial decomposition, all of which can decrease the amount of oxygen available in the water. Sewage is the main source of excess nitrate added to the water. Fertilizers and agricultural runoff also contribute high levels of nitrate. Nitrates can be harmful to humans because it affects the ability of our red blood cells to carry oxygen. This is especially true for infants or people with already weak immune systems. Directions: 1. Fill the test tube to the 5mL line with your water sample. 2. Add 1 nitrate test tab. 3. Cap tube and mix until the tab has dissolved. 4. Wait 5 minutes for the color to develop. 5. Compare the color of the sample to the Nitrate Color Chart (if no color develops at all, the result is 0ppm). Record the ppm Nitrate. 6. Determine the nitrate Q-value of your sample. ppm Nitrate 0 5 20 40 Q-value 98 95 73 36 Phosphate Test Phosphate is a nutrient needed for plant growth and animal growth. It is also a fundamental element in metabolic reactions. High levels of this nutrient can lead to the overgrowth of plants, increased bacterial activity, and decreased oxygen levels. Phosphate comes from several sources, including human and animal waste, industrial pollution, and agricultural runoff. Phosphates can be present in the water in many forms, so this test will give an estimate of the total amount of phosphate potentially available in a given water supply. Directions: 1. Fill the test tube to the 10 mL line with your water sample. 2. Add 1 phosphorus test tablet. 3. Cap and mix tube until the tablet has dissolved. 4. Wait 5 minutes for the blue color to develop. 5. Compare the sample to the phosphate color chart (if no color develops at all, the result is 0ppm). Record the ppm Phosphate. 6. Determine the nitrate Q-value of your sample. ppm Phosphate Q-value 0 98 1 92 2 37 4 14 pH Test pH is a measurement of the acidic or basis quality of water. Most forms of aquatic life tend to be very sensitive to changes in pH. These organisms are highly adapted to a specific pH level and may die if the pH of water changes even slightly. The pH of natural water is usually between 6.5 and 8.2. The pH of seawater is usually between 7.8 and 8.4. Directions: 1. Turn on palm and open up the Imagi Probe Software. 2. Make sure it is on Investigation 4 3. Press New Trial and make sure the sensor is set to test pH. 4. Rinse probe with Distilled water from Squeeze bottle and wipe dry with a Kim Wipe. Place probe in sample. 5. Press Preview on Palm, then press Collect 6. The probe will collect 5 pH readings during one minute and display them (the red numbers in the upper left). Record the 5 pH readings on your data sheet. Take the average and record this as the pH reading for your sample. 7. On the Palm, press Stop, Cancel, and then Delete to prepare the probe for the next group. 8. Compare to the pH Water Quality Values chart to determine the Q-value for your sample. pH Water Quality Values PH <2.0 2.0 3.0 4.0 5.0 6.0 7.0 7.5 8.0 9.0 10.0 11.0 12.0 >12.0 Q-value 0 2 4 8 24 55 90 92 85 50 50 22 7 2 Percent Saturated Dissolved Oxygen Temp oC (unbolded numbers are the % saturation for the associated ppm Dissolved Oxygen ppm and Temp) 0 ppm 4 ppm 2 0 29 4 0 31 6 0 32 8 0 34 10 0 35 12 0 37 14 0 39 16 0 41 18 0 42 20 0 44 22 0 46 24 0 48 26 0 49 28 0 51 30 0 53 8 ppm 58 61 64 68 71 74 78 81 84 88 92 95 99 102 106 % Saturated DO Water Quality Values % Saturation 0 30 40 50 60 70 80 90 100 110 Q-value 0 20 30 43 56 77 88 95 100 95 Water Quality Index Data Sheet Circle letter of your sample: A B C D E Group Members: Test Parameter Test Results Q-value Total Weighing Quality Factor* Index Temperature: Dissolved ppm DO: Oxygen % Saturation: Positive Fecal Coliform Negative 0.27 0.25 Nitrates ppm Nitrate: 0.16 Phosphates ppm Phosphate: 0.16 pH pH1: pH2: pH3: pH4: pH5: Average pH: 0.17 *The weighing factor indicates the importance of each parameter to the overall water quality. The parameters with higher weighing factors are considered more important in determining water quality than the ones Circle the Water Quality with smaller factors. of this sample based on index Overall Water Quality Index:_______ (Sum up values in last column) (use scale): Guess where your sample came from: Actual Location Where Sample Taken: Excellent Good Medium/Average Fair Poor (Ask mentor)