Chapter 15 notes
advertisement

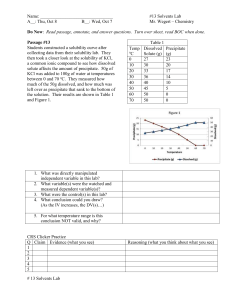
Chapter 15- Solutions and Colloids Solutions: homogeneous on a molecular level Colloids: homogeneous on a macroscopic level Suspensions: heterogeneous mixture that can separate ex: ex: ex: 1. does NOT settle out 2. PASSES through a filter paper 3. PASSES through a membrane 4. does NOT scatter light 5. DOES affect colligative properties 1. does NOT settle out 2. PASSES through a filter paper 3. SEPARATED by a membrane 4. SCATTERS light 5. does NOT affect colligative properties 1. settles out on standing 2. SEPARATED by ordinary filter paper 3. SEPARATED by a membrane 4. SCATTERS light 5. does NOT affect colligative properties Characteristics of Colloids: •composed of two phases 1. dispersed phase (what's mixed in) 2. continuous phase(what does the mixing) •e.g.'s emulsions, aerosols, foams, & gels colloidal particles •too small to see with a microscope •but can be seen with an electron microscope or by using the Tyndall effect. Tyndall effect •light is scattered by colloidal & suspended particles Brownian motion •random motion of particles caused by their temperature (their kinetic energy) •random motion of larger particles being bombarded by water molecules Properties of Solutions: solute is dissolved in the solvent. “Hide your loot in the vent!” Types of Solutions: (Fig 15.3 on p.503) Gas in gas, gas in liquid, liquid in liquid, solid in liquid, gas in solid, liquid in solid, solid in solid CONCENTRATION OF SOLUTIONS: Concentration is the amount of solute in a given amount of solvent. Molarity (M) Molarity moles of solute liters of solution M mol L lab equipment: volumetric flask 1. Vinegar is a solution of acetic acid. What is the molarity of the solution produced when 125 g of acetic acid (C2H4O2) is dissolved in sufficient water to prepare 1.50 L of solution? 2. How many grams of bromine are needed to prepare 0.500 L of a 0.0100 M solution of bromine in water? Molality (m) molality m moles of solute mol ki log rams of solvent kg Molarity (M) uses volume of solution, whereas molality (m) uses kilogram of solvent. Molality is independent of temperature. 3. What is the molality of a solution containing 125g of iodine and 750. g of carbon tetrachloride (CCl 4)? 4. What is the molality of a solid solution containing 1.576 g of iron and 0.0021 g of lead? Mole Fraction (X): moles of component mole fraction total moles of solution X mol part mol whole The sum of all mole fractions within the same solution equal to one. 5. A gas mixture contains 50.4g of dinitrogen monoxide and 65.2 g of oxygen gas. What is the mole fraction of dinitrogen monoxide? 6. A gas mixture contains the following gases with the mole fractions indicated: N 2 (0.450), O2 (0.334), CO2 (0.023), SO2 (0.017), and N2O4 (0.120). The mixture also contains the gas argon. What is the mole fraction of argon? Formation of solutions: Solvation: the surrounding of solute particles by solvent particles Hydration is solvation when water is the solvent. Intermolecular forces are broken during hydration and new attractions between the solute and solvent are formed. This breaking and forming of attractions may lead to exothermic or endothermic processes. Dissociation: crystals break into ions during hydration -Ionic cmpds, salts, acid & bases all dissociate to form electrolytic sol’ns. (conduct electricity) -covalent cmpds tend not to dissociate. Solubility: the amount of solute that will dissolve in a specific solvent under given conditions. (grams of solute/100 g of solvent at a specific temperature and pressure) Miscibility: “like dissolves like” (soluble) miscible not solubleimmiscible polar dissolves polar nonpolar dissolves nonpolar Solubility- how much you can dissolve -saturated vs. unsaturated, supersaturated -soluble: a solute dissolves readily -insoluble: a solute does not readily dissolve FACTORS AFFECTING SOLUBILITY: 1. Temperature as temperature increases, solubility increases -supersaturated sol’n -for gas dissolved in liquid, as T increases, solubility decreases, ex: soda 2. Pressure as pressure increases, solubility increases. (soda), Henry’s Law-solubility of a gas is proportional to the partial pressure of the gas above the liquid. Factors affecting rate of solubility- based on solvation (bringing new particles of solvent to surround solute) 1. Surface Area: 2. Agitation as surface area increases, solubility increases, as particle size decreases, solubility increases as stirring increases, solubility increases Colligative Properties: Colligative properties-are determined by the number of solute particles in solution rather than the type of particle in solution 1. Vapor Pressure Reduction Raoult's law -The vapor pressure of a solvent is inversely proportional to the number of solute particles •adding a solute to a solvent will lower its vapor pressure because the solute molecules take up space at the surface of the liquid and prevents some solvent molecules from leaving the liquid. 2. Boiling Pt. Elevation Due to the lowering of the vapor pressure, boiling point will increase because a higher temperature is necessary to get the vapor pressure of the solution up to atmospheric pressure so that the solution boils. Ex. Adding salt when cooking pasta, coolant (antifreeze) in your car engine Tb = Kb m 7. What is the boiling point of water if 100. g of sucrose, C12H22O11, is added to 500. g of water? (Kb for water is 0.52 Co/m) 3. Freezing Pt. Depression Due to the lowering of the vapor pressure, the freezing point will decrease because the freezing point of a substance is the temperature at which the vapor pressures of the solid and liquid phases are the same. Ex. Salt on icy roads, antifreeze (ethylene glycol), making ice cream Tf = Kf m 8. Calculate the freezing point of a solution of 100. g of ethylene glycol (C2H6O2) antifreeze in 0.500 kg of water. (Kf for water is 1.86 Co/m) Note ionic compounds dissociate into multiple ions Therefore, you need to calculate the effective molality of a solution. Multiple the molality by the # of ions the compound splits into. NaCl(s) ---> two dissolved particles AlCl3(s) ---> four dissolved particles MgCl2(s) ---> three dissolved particles Al2S3(s) ---> five dissolved particles 9. Calculate the boiling point when 153 g of NaCl are added to a pot of 8 kg of water. 4. Osmotic PressureOsmosis is the net flow of solvent (water) from the less concentrated solution to the more concentrated solution. Osmosis happens across a semi-permeable membrane that allows small particle such as water to move but not larger molecules. osmotic pressure- the pressure difference between two sides of a semipermeable membrane Ex. Fluids given intravenously (IV) must be isotonic (same osmotic pressure) as blood. RBC in hypotonic solution will burst (hemolysis)- net diffusion into RBC RBC in hypertonic solution will shrivel (crenation)- net diffusion out of RBC Ex. Eat salty foods bloated As T increases, solubility increases for solids dissolved in liquids. As T increases, solubility decreases for gases dissolved in liquids. The curve represents the maximum amount of solute that can be dissolved in 100 g of H2O for that temperature saturated solution. Above the curve supersaturated solution Below the curve unsaturated solution If I have 80 g of NaNO3 dissolved in 100 g of H2O at 100C, what type of solution do I have? If I increase the temperature to 20oC, what type of solution do I have? If I decrease the temperature to 0oC, what type of solution do I have? Why are the curves negative in slope for NH3 and HCl? Electrolytic solutions: Ionic compounds and polar substances dissolved in water: Salt water, vinegar, tap water But not distilled water, sugar water and oil.