3_5_Light Energy and Colors Walkthrough
advertisement
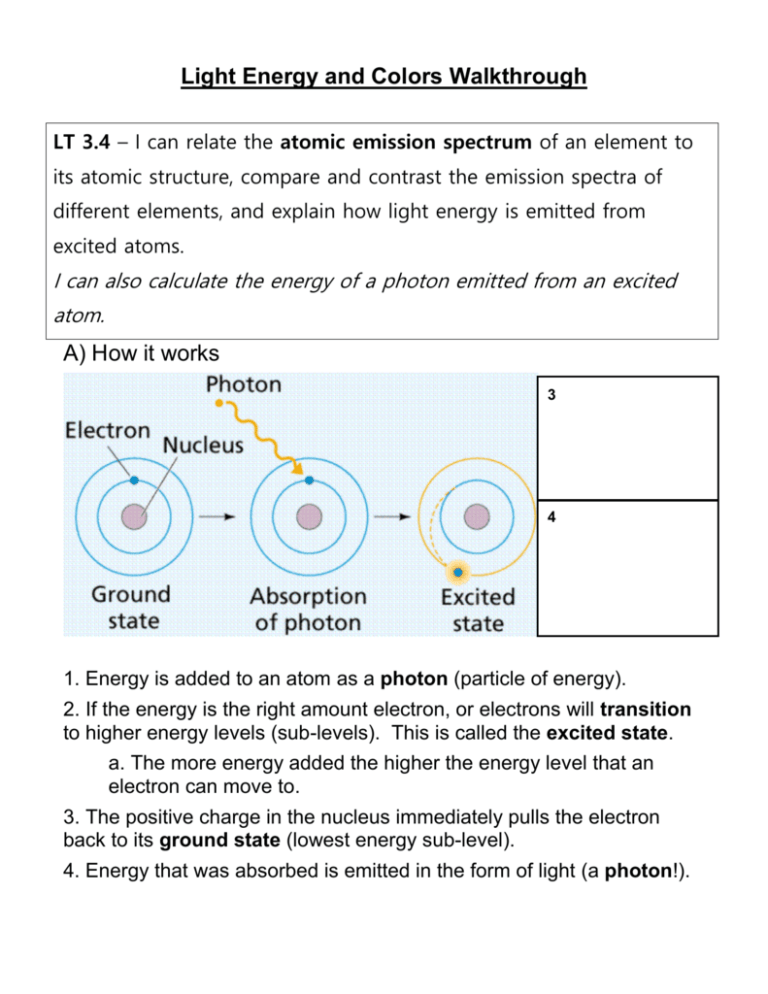
Light Energy and Colors Walkthrough LT 3.4 – I can relate the atomic emission spectrum of an element to its atomic structure, compare and contrast the emission spectra of different elements, and explain how light energy is emitted from excited atoms. I can also calculate the energy of a photon emitted from an excited atom. A) How it works 3 4 1. Energy is added to an atom as a photon (particle of energy). 2. If the energy is the right amount electron, or electrons will transition to higher energy levels (sub-levels). This is called the excited state. a. The more energy added the higher the energy level that an electron can move to. 3. The positive charge in the nucleus immediately pulls the electron back to its ground state (lowest energy sub-level). 4. Energy that was absorbed is emitted in the form of light (a photon!). STOP AND JOT FIRST: Label the diagram with #1 and #2 THEN: Draw #3 and #4 in the box next to the diagram. B) The nature of light 1. Visible light is a small part of the electromagnetic spectrum. (Circle visible part of spectrum) Roy G. Biv We see only 1.5% of entire spectrum….Unless you’re the predator! https://www.youtube.com/watch?v=Y1txEAywdiw What would happen if we saw everything? http://www.chemistryland.com/CHM107Lab/Exp7/Spectroscope /Spectroscope.html 2. Light is known to have wave-like properties of frequency (ν) and wavelength (λ). a. Wavelength (λ) – the distance from wave crest to crest. b. Frequency (ν) – the number of waves per second, measured in hertz (Hz) Label wavelength in the 3 diagrams above What is the frequency of the 1st wave? The 2nd wave? The 3rd? 3. Different light has different wavelengths, which determines the amount of energy of that light. a. Determining wavelength Ex. Convert 750 nm to m b. Calculating E 1st Step: Figure out frequency ν = c/λ where: ν = frequency in 1/s c = speed of light = 3.00 x 108 m/s λ = wavelength of light in m 2nd Step: Use ν to figure out energy Ephoton = hν where: E = energy in Joules (J) h = Planck’s constant = 6.626 x 10-34 J*s ν = frequency in 1/s I DO Calculate the energy of a photon released from an atom with a wavelength of 600 nm. WE DO The laser used to read information from a compact disk has a wavelength of 780 nm. What is the energy associated with one photon of this radiation? Wavelength (nm) 600 750 Energy (J) As wavelength increases, energy ___________. As wavelength decreases, energy __________. 780 C. Spectroscopes Atomic emission spectra 1. Explanation: Multiple bands are seen as the electron moves between a range of specific energy levels. 2. We can calculate the energy of these bands because they fall on a scale that measures wavelength (λ) of that particular band of light.










