Molecular Polarity:
advertisement
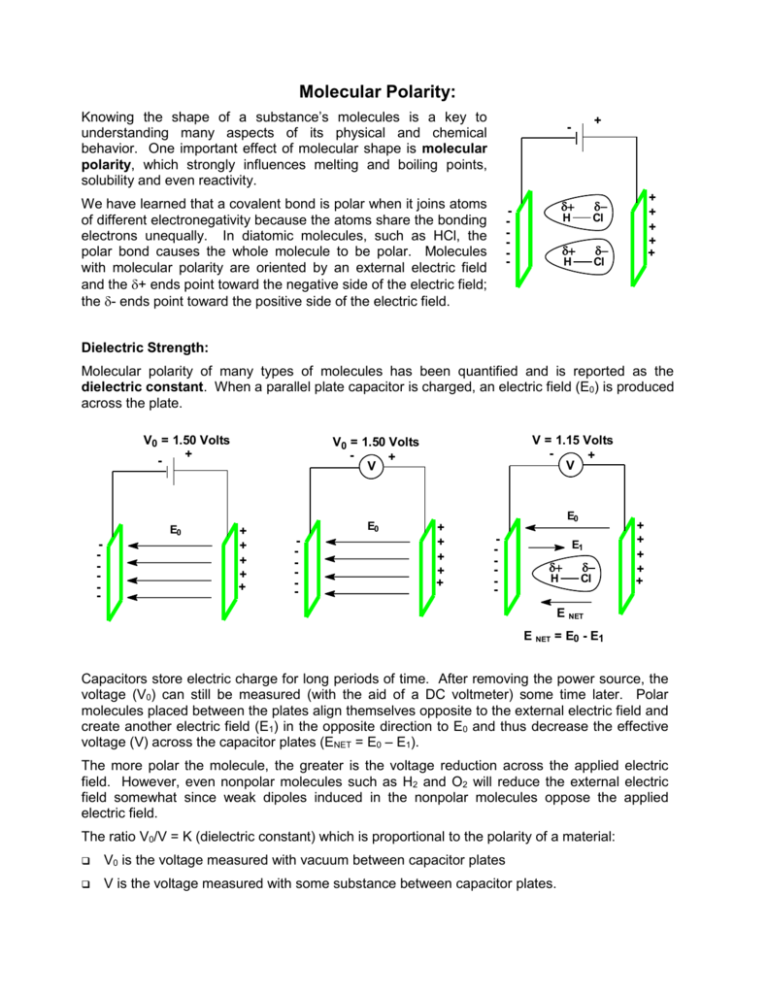
Molecular Polarity: Knowing the shape of a substance’s molecules is a key to understanding many aspects of its physical and chemical behavior. One important effect of molecular shape is molecular polarity, which strongly influences melting and boiling points, solubility and even reactivity. + - We have learned that a covalent bond is polar when it joins atoms of different electronegativity because the atoms share the bonding electrons unequally. In diatomic molecules, such as HCl, the polar bond causes the whole molecule to be polar. Molecules with molecular polarity are oriented by an external electric field and the + ends point toward the negative side of the electric field; the - ends point toward the positive side of the electric field. - + - H Cl + - H Cl + + + + + Dielectric Strength: Molecular polarity of many types of molecules has been quantified and is reported as the dielectric constant. When a parallel plate capacitor is charged, an electric field (E0) is produced across the plate. V0 = 1.50 Volts + - E0 - V = 1.15 Volts + V V0 = 1.50 Volts + V + + + + + E0 - + + + + + E0 - E1 + - H Cl + + + + + E NET E NET = E0 - E1 Capacitors store electric charge for long periods of time. After removing the power source, the voltage (V0) can still be measured (with the aid of a DC voltmeter) some time later. Polar molecules placed between the plates align themselves opposite to the external electric field and create another electric field (E1) in the opposite direction to E0 and thus decrease the effective voltage (V) across the capacitor plates (ENET = E0 – E1). The more polar the molecule, the greater is the voltage reduction across the applied electric field. However, even nonpolar molecules such as H2 and O2 will reduce the external electric field somewhat since weak dipoles induced in the nonpolar molecules oppose the applied electric field. The ratio V0/V = K (dielectric constant) which is proportional to the polarity of a material: V0 is the voltage measured with vacuum between capacitor plates V is the voltage measured with some substance between capacitor plates. Good dielectrics are polar and are good electrical insulators. They lower a capacitor’s voltage. The dielectric constant (K) is a dimensionless ratio. The higher the dielectric constant of a substance, the greater is its polarity. Hydrogen bonded substances, e.g., H2O, HF, NH3 have unusually high dielectric constants since they are especially good electric insulators. Some values are listed below. Name Formula dielectric constant (K) mp (C) bp (C) (g/mL) formamide HCONH2 110 3 193 1.13 sulfuric acid H2SO4 101 10 270 1.83 hydrogen fluoride HF 84 -83 20 1.002 water H2O 81 0 100 0.998 dimethyl sulfoxide (DMSO) (CH3)2SO 47 18 189 1.10 dimethylformamide (DMF) HCON(CH3)2 37 61 152 0.945 acetonitrile CH3CN 36 -45 82 0.79 methanol CH3OH 33 -94 65 0.79 hexamethylphosphoramide HMPA 30 7 235 1.03 ethanol CH3CH2OH 24 -117 78 0.79 ammonia NH3 23 -78 -33 0.69 dimethyl ether CH3OCH3 21 -95 56 0.79 dichloromethane CH2Cl2 9 -97 40 1.42 tetrahydrofuran THF 7.6 -65 66 0.89 acetic acid CH3COOH 6.2 -17 118 1.04 chloroform CHCl3 4.8 -64 62 1.48 hydrogen iodide HI 3.6 -51 -35 -- benzene C6H6 2.3 5 80 0.88 hexene C6H12 2.2 7 81 0.78 n-hexane C6H14 2 -95 69 0.66 carbon tetrachloride CCl4 2 -23 77 1.59 Note that a higher a dielectric constant implies relatively high mp and bp. Molecular weight is also a large factor-all else being equal, mp and bp points increase with molecular weight. As we shall see in the next unit, larger molecular weights give rise to stronger intermolecular forces of attraction and hence higher mp and bp. Dielectric Constant and Solubility: Water is often referred to as the ‘universal solvent’ in that it dissolves so many substances but it is by no means truly a universal solvent. Water dissolves many ionic compounds (e.g., NaCl), polar compounds (e.g., sugar) but relatively few nonpolar compounds (e.g., gasoline, C8H18). We can explain the solvent power of water in terms of its ability to reduce attractive forces between oppositely charged species. Since water has a high dielectric strength, we know that it is a good electric insulator. The larger dielectric constant of a solvent, the smaller will be the solute-solute attractive forces. Small highly polar molecules (e.g., H2O) interject themselves between positive and negative ions in solution. The negative pole of the H2O molecule (oxygen atom) points to the solute cation, while the positive poles of the H2O molecule (hydrogen atoms) point to the solute anion. + + H + - H H :O : : :O - - H + + : :O H H H Cl - - :O : : :O H + + H + H + :O : H H + + - H - : O: H H K+ + + + + H + O: : - In this way water molecules effectively insulate the ions from one another, thereby making it easier for the solute to pass from the solid state into solution. Many polyvalent ions, e.g., Cu+2 dissolve exothermically in H2O forming hydrated ions, e.g., Cu(H2O)4+2. In many cases the water molecules accompany the cations when solids are crystallized from aqueous solutions, i.e., water of crystallization, e.g., MgCl26H2O, Fe(NO3)26H2O, Fe(SO4)27H2O (an additional H2O molecule is associated with SO4-2 anion), KAl(SO4)212H2O [(alum) – each cation accommodates six H2O molecules]. Attraction between negative ions and water is not nearly as important as between positive ions and water. Anions are generally larger than cations so their charge is more dispersed and thus anion association with H2O is weaker. Solvent-Solvent Interactions: Liquids having similar dielectric constants (e.g., H2O and acetonitrile) are likely to be miscible (soluble in all proportions) while liquids having very different K values (e.g., H 2O and hexane) are normally immiscible (insoluble in each other). The general rule is ‘like dissolves like’. Dipole Moment: Another method of expressing molecular polarity is called dipole moment. Like dielectric strength, the dipole moment is determined by measuring the voltage changes between electrically charged plates when various substances are placed between the plates. The results are expressed differently in the case of dipole moments. Because a polar bond has a + and - end, it has a dipole. The size of the dipole is quantified by the dipole moment () which is defined as the product of the magnitude of the charges separated (q) times the distance by which they are separated (d), i.e., the distance between the centers of positive and negative charge. d . dipole moment, = q d + - dipole units =debye (D) 1 debye = 3.333 x 10-30C.m (C . m =coulomb meters) Because the charge on an electron is 1.60 10-19 coulombs and the distance between charges in a polar bond is in the order of 10-10 m, the product of charge times distance ( = qd) is in the order of 10-29 Cm. Example: HCl has a bond length of 1.274 10-10m and its dipole moment is measured as 1.08D. Calculate the charge separation: 1.08D 3.33 10 -30 C m D 2.82 10 - 20 C -10 d 1.274 10 m 2.82 10 -20 C This quantity of charge is 100% 18% of the charge of an electron. 1.69 10 -19 C/e qd q Thus in HCl, chlorine has an excess of .18 electrons (-) and hydrogen has a deficit of .18 electrons (+). The dipole moment (debyes) of some bonds are listed. H-C 0.4 H-Br 0.8 C-F 1.4 H-N 1.3 H-I 0.4 C-Cl 1.5 H-O 1.5 C-C 0 C-Br 1.4 H-F 1.7 C-N 0.2 C-I 1.2 H-Cl 1.1 C-O 0.7 bond bond length (m) EN dipole moment (D) H-F 0.92 10-10 1.9 1.7 H-Cl 1.27 10-10 0.9 1.1 H-Br 1.41 10-10 0.7 0.82 H-I 1.61 10-10 0.4 0.44 In a molecule with only on covalent bond, e.g., HCl, the dipole moment of the bond is also the dipole moment of the whole molecule. The dipole moment of a molecule with more than one covalent bond must be the vector sum of all bond dipoles. .. H :O : Cl C C O .. Cl H H Cl Cl H H H C N = 3.9 D acetonitrile : H N H H = 1.5 D ammonia .. CHCl3 = 1.9 D = 1.0 D H3C H2O = 1.9 D .. C .. H Cl CH3Cl O : Cl H H = 0.0 D H C C O .. = 0.0 D = 1.7 D C C CO2 CCl4 CH3OH H .. .. H H : Cl : H3C O .. acetone = 2.9 D Cl Lone pairs of electrons also contribute to the dipole moments of molecules since they correspond to charge separation with the nucleus having a + charge balanced by the - charge of the lone pair of electrons. The contribution of the lone pairs helps explain the large dipole moments of acetone and acetonitrile. The dipole moments (in debyes) of some molecules are listed. H2 0.0 H2O 1.85 Cl2 0.0 NaCl 9.0 BF3 0.0 CsCl 10.4 CO2 0.0 PH3 0.58 CH4 0.0 AsH3 0.20 CH3Cl 1.87 SbH3 0.12 CH2Cl2 1.55 CO 0.12 CHCl3 1.02 O3 0.53 CCl4 0.0 cis-CHCl=CHCl 1.90 NH3 1.47 trans-CHCl=CHCl 0.0 NF3 0.24 Problem: Explain he difference in the dipole moment of NH3 and NF3.








