DRUG-_039_Pharmaceutical_Sector
advertisement

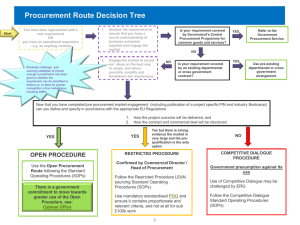
DRAFT Situational Analysis Pharmaceutical Sector in Cambodia Table of Contents Introduction ......................................................................................................................... 1 Context ................................................................................................................................ 1 1. Pharmaceutical control.................................................................................................... 2 1.1 The Department of Food and Drugs, Ministry of Health.......................................... 2 1.1.1. Registration and Cosmetics Bureau .................................................................. 2 1.1.2 Essential Drugs Bureau ...................................................................................... 2 1.1.3 Narcotic Control and Pharmaceutical Trade Bureau ......................................... 3 1.1.4 Drug Regulation Bureau .................................................................................... 3 1.2 Procurement of pharmaceuticals ............................................................................... 3 2. Progress to date ............................................................................................................... 5 3. Major issues .................................................................................................................... 6 3.1 DDF and the private sector ....................................................................................... 6 3.2 Unregistered drugs .................................................................................................... 6 3.3 Counterfeit drugs ...................................................................................................... 6 3.4 Unlicensed drug sellers ............................................................................................. 6 3.5 Strategic Planning ..................................................................................................... 7 3.6 Prescribing practices and irrational use .................................................................... 7 3.7 Lack of knowledge .................................................................................................... 7 3.8 Procurement and supply system................................................................................ 7 3.9 Registration process .................................................................................................. 7 3.10 Training of pharmacists .......................................................................................... 7 3.11 Inspections .............................................................................................................. 8 3.12 Prosecution of illegal drug stores ............................................................................ 8 Introduction This report provides an assessment of the pharmaceutical situation in Cambodia and identified key issues. For people in Cambodia, like those in many developing countries with a large rural population, drugs are often the first and only access to modern medicine. Most access drug through a poorly regulated private sector as the public lacks of health personnel and infrastructure. Several problems related to pharmaceuticals limit even this minimum access to the medicines necessary. The Department of Drugs and Food in the Ministry of Health is responsible for the quality and control of pharmaceuticals in Cambodia. For the purposes of this work, Food Safety Bureau in the Department will not be considered. Context Years of internal strife have had a major impact on the pharmaceutical sector in Cambodia. During the Khmer Rouge years, the country lost most of its pharmacists and pharmacies. Prior to this strife, pharmaceutical laws and regulation were well enforced, and only over-the-counter drugs could be bought without a prescription. However, since the period of civil unrest and the introduction of a more open market prescriptions are no longer needed to obtain pharmaceuticals. Most drugs can be purchased over the counter and most people choose to obtain drugs directly from drug stores rather than consulting doctors. There are three legal outlets for selling drugs in Cambodia i.e. outlets that are licensed. a) A pharmacy-run by pharmacist b) A depot A-run by assistant pharmacist (three years of training) c) A depot B-run by a “retired” nurse However, there are a large number of illegal drug sellers, 81% of drug shops in the provinces are unlicensed and 71% of all drug shops are unlicensed. The table below shows the number of licensed and unlicensed drug outlets. Population Pharmacy Depot A Depot B Illegal stores Total: Province 10,700,000 129 95 334 2394 Total: Cambodia 11,700,000 393 175 446 2461 The above “Illegal drug stores” only include large stores that openly sell drugs. Unlicensed drug sellers are often the only providers of drugs in rural areas. 1. Pharmaceutical control 1.1 The Department of Food and Drugs, Ministry of Health The Food and Drug Department in the Ministry of Health is the government agency responsible for pharmaceutical control in Cambodia. The department is led by a Director who reports directly to the Director General of Health. The Director has overall responsibility for the department and also has responsibilities in the School of Medicine. There is one Vice Director whose role is to support the Director. It has five bureaus: 1) Registration and Cosmetics Bureau 2) Essential Drugs Bureau 3) Pharmaceutical Trade Bureau 4) Drug Regulation Bureau 5) Food Safety Bureau 1.1.1. Registration and Cosmetics Bureau The Registration and Cosmetics Bureau is responsible for registering all drugs and specific cosmetics coming into the country. Registration is the main mechanism for controlling the quality of pharmaceutical in the country. It involves evaluating documents on the following criteria: o Administrative-application, summary of product info, GMP, CPP (certificate of pharmaceutical product) o Quality- qualitative quantification formulation, manufacturing process, certificate of analysis of ingredients, control procedures of ingredients, stability, bioavailability and bio-equivalence o Pre-clinical-pharmaco-kinetics, toxicology o Clinical trials Activities in this bureau are supported by the national budget and WHO 1.1.2 Essential Drugs Bureau The Essential Drugs Bureau Chief is responsible for rational drug use, for ensuring adequate supplies available in the public sector, and for improving the quality of clinical pharmacology. Its main functions are to: Provide support, supervision and training in ODs, referral hospitals, and health centers, inspect expired drugs in provinces; Redistribute pharmaceuticals from over-stock units to out of stock facilities to ensure access. Keep central records for 72 ODs of pharmaceutical supplies in a central data base Translate information documents from WHO and other sources and produce quarterly bulletin, which are distributed to all public sector health facilities. Prepares posters, TV spots, and radio broadcasting related to rational and safe pharmaceutical drug use. . This bureau receives supported from the national budget, UNICEF, RACHA, KFW, WHO, and WB. 1.1.3 Narcotic Control and Pharmaceutical Trade Bureau Narcotic Control and Pharmaceutical Trade Bureau licenses pharmaceutical companies in collaboration with the Ministry of Commerce. It also Registers import/export companies Publishes drug import permit following the international convention on narcotics control ????? Registers traditional medicine The activities in the bureau are supported by national budget. 1.1.4 Drug Regulation Bureau The Drug Regulation Bureau is responsible for drug legislation. Its main functions are to: Develop drug legislation and regulation Produce policy and guidelines such as the rational drug use policy, GMP guidelines Inspect manufacturing plants, drug import and export companies and drug retail outlets (pharmacies) Advertise licenses, translate drug leaflets into Khmer, and control drug advertisement Oversee the administration, documentation, and security of department The activities of this bureau are supported by the national budget. The donor support currently available to the Department of Drugs and Food suggests significant gaps in funding to drug regulation. 1.2 Procurement of pharmaceuticals The availability of drugs at each health facility, from referral hospitals to health centers, is one of the most critical elements of a successful health sector in Cambodia. The responsibility for the procurement of pharmaceuticals must take into account quality, cost, and timely supply. The pharmaceutical “market” has unique characteristics that require expert knowledge over and above that needed for the procurement of other items. The structure of drug procurement in Cambodia has unstable history. From 1994-1995 the procurement of pharmaceuticals was managed by the Department of Drugs and Food. In 1995-1998 procurement was managed by a MoH procurement committee but 19982003 it was transferred to a unit in the Department of Budget and Finance. This unit worked as MoH procurement bureau to prepare tendering documents. In 2003,a sub-degree initiated by the MoEF shut the procurement bureau and established a procurement committee that is independent from the existing Departments and Bureaus at MoH. The procurement committee members all have another position in Department of Budget and Finance. Its establishment appears to be linked with a decision to introduce an open tendering process aimed to improve procurement prices through competitive bidding. . The PEAC= Pre-qualification Evaluation and Awards Committee has been established since 1995. The PEAC is responsible for overseeing the procurement of pharmaceuticals. Final decision is made by PEAC, not the procurement committee. It membershipare senior officials from the MoH and MoEF. The current members are: o Ex. Eng Huot (Director General of Health) o Ex. Ung Phirum (Secretary of State) o Ex. Te Huy Sieng (Director General of Administration and Finance) o Mr. Chea Kim Long (Director of Department of Finance) o Mr. Bun Leang Heng (Chief of Cabinet) o Mr. Nop Yim (Chief of the Procurement Committee); and o A controller from MEF This committee does not include a person with “technical expertise” in drug procurement. Within the MoH the structure and process for ordering drugs is: The Department of Drugs and Food provide a list of drugs and quantities needed based on prior use. They also provide information on the most cost effective drugs available to the MoH procurement committee. The MoH procurement committee: Estimates the total cost of the drugs needed Seeks MoEF approval of the budget Prepares the bidding documents and tender, and Selects the suppliers The MoEF and provides final approval of the tender documents and suppliers. In 2002 the MoEF made decisions, with little technical input from the MoH that resulted in significant delays in drug procurement. In an environment of decentralization, this level of decision-making after the budget has been approved is difficult to understand, especially when pre-selection of pharmaceutical providers requires considerable technical knowledge. There has been serious shortages of drugs in the public health system. In August 2003 only 40% of drugs bought were in the central medical store (CMS) when usually 100% of all drugs for a year should be available by June. The “open tender” process that has been introduced is in fact very restrictive. Although in theory it is an open tendering process a number of official and un-offical rules severely restrict it. The tender documents are provided and prepared in Khmer only. This restricts non Khmer firms from bidding and defeats the purpose of open tendering. There is a lack of clarity and transparency in the tender criteria. Payment is made after delivery and there is often a delay in payments. There is no open letter of credit, and it is unclear what current payment can be made in. These “rules” or tendering criteria restrict the number of companies that would be interested in bidding. For instance, it is reported that in one instance a successful bidder did not contact the pharmaceutical companies before submitting tender. It was only after the company was selected that it started contacting the companies to provided the drugs. Most companies could not provide at the tender price. Issues of quality and delivery time were not addressed by the successful bidder. There is no pre-qualification for the bidders therefore, reliable suppliers of high quality products with experience in procurement were not ensured. There is poor collaboration between the Ministry of Economics and Finance and the Ministry of Health and an apparent unwillingness to use technical support available in the MoH to assist the procurement process. For instance, in 2002, MoH selected 5 reputable companies but only one of these companies was by approved the MEF. 2. Progress to date The Ministry of Food and Drugs has made solid progress in pharmaceutical control over the last 10 years. There have been improvements in the quality of drugs available in the country. The number of pharmacists has increased XXXXXXXXX There has been an improvement in the supply system the public health facilities. An efficient system of collecting pharmaceutical data is in place. A computerize supply management system has been introduced. There has been a considerable amount of in-service training in XXXX There has been a significant improvement in rational drug use in some referral hospitals XXXX A drug registration system has been introduced and staff have received training. Fifty precent (50%) of drugs in the country are registered. The Malaria, TB, HIV/AIDS programmes are actively involved in ensuring there are good practice guidelines and an adequate supply of quality drugs so that treatments is available for these major diseases. What percentages of patients have access to treatment? XXX Ask Jay,Reiko and Veronique There are good relationships between the Essential Drug Bureau, WHO, KFW, UNICEF, and RACHA. Each fund specific and different activities to support essential drugs management Regular meeting with partners are held at central level. Coordination of donor funding is reported to be improving. Most provinces have just had computers installed so that the drug supply system can be computerized. Training is in progress. 3. Major issues 3.1 DDF and the private sector Eighty percent (80%) of the people purchase drugs in the private sector. The Department of Food and Drugs has few activities focused on the private sector. It receives no donor support to increase the licensing of pharmaceutical outlets or to influence the behavior of vendors. 3.2 Unregistered drugs Only 50% of drugs in the country are registered. There is a backlog of drugs waiting for registration. More than 3000 un-registered drugs can be found in the market. Illegal importing is a problem. Cambodia has neighboring countries bordered, and the drugs can be smuggled easily, especially from Vietnam and Thailand. Some companies don’t have import license, but have found ways to avoid customs control. Inspection are not carried out regularly of drug store, importing companies, and manufacturer 3.3 Counterfeit drugs There is a significant problem with counterfeit drugs in the country. One study demonstrated a high distribution (25% to 38%) of fake artesunate, a key antimalarial drug in the treatment of multi-drug resistant malaria. Counterfeit artesunate aggravates the problems of worsening drug-resistant malaria in the region. The majority of the population is unable to distinguish between a legitimate and counterfeit drug, and it is sometimes even difficult for trained pharmacists to distinguish the differences due to their high resemblances to the original “real” drugs. 3.4 Unlicensed drug sellers Seventy percent (70%) of the population use the private sector , most of which is unregulated. The poor tend to use unlicensed drug vendors because it is easier and faster than the public sector (where they provide drugs without charge), and in the current system, they often have to pay for drugs and consultation anyways. Therefore the poorest people often do not get treatment at the public health facilities. Unlicensed drug sellers are the reality, and they sell most drugs in the country. Because they are unlicensed there is a reluctance to work with them to improve their service. One NGO has elected to train unlicensed drug sellers in rational drug use, but they are facing criticism for working with the “illegal drug sellers.” 3.5 Strategic Planning There is no strategic planning for the pharmaceutical sector to focus donor contributions where the need is greatest. Most donors support activities in the Essential Drugs Bureau, whereas many major problems are related to procurement and regulation. 3.6 Prescribing practices and irrational use Prescribing practices and pharmaceutical use in Cambodia is inappropriate both from the supply and demand side. Poly-pharmacy: e.g. prescription for 15 drugs for one patient is a common practice. There is abuse of use of injections and antibiotics, poor patient compliance, and no prescription required to purchase drugs. Most people go directly to the pharmacy to avoid consultation fee from the doctors. 3.7 Lack of knowledge Drug providers themselves have limited knowledge on rational drug use and the high illiteracy rate in the country means that much of the population is unable to read or understand labels on medicines. There is a lack of training of drug sellers. There is inappropriate health seeking behavior and lack of knowledge among the population about the appropriate use of pharmaceuticals. 3.8 Procurement and supply system In addition to problems resulting from the current procurement structure there are some longer-term problems associated with procurement. There are frequent stock-outs at CMS, OD pharmacies, referral hospitals, and health centers. The exchange of information from provinces to the central level is slow, there is poor forecasting, procurement is only done once a year by the central team, and there is over-stocking (due to poor forecasting) that leads to expired drugs. The current quantity of drugs ordered is calculated from the past consumption of CMS and each OD, which does not take into account changes in requirements. The centralized ordering and delivery makes precise forecasting cumbersome. Requests from PHD are delayed and inconsistent so rough estimates have to be made. 3.9 Registration process The process for registration of drugs is limited. The laboratory facilities have low capacity to test quality so registration is based on documentation only and work from other countries. 3.10 Training of pharmacists Only a limited number of pharmacists exist????? Most pharmacists and legal drug sellers tend to work in cities, and not in the rural regions. Responsibility of drug sellers is low. They do not necessarily think of what are the good drugs, what is the right quantity, but focus more on what is profitable. 3.11 Inspections Low public service salaries and lack of funds to transport and support costs together with an attitude of “no work if there is no incentives” means there is limited inspection of drug vendors. 3.12 Prosecution of illegal drug stores It is illegal to open a drug store without license and the law provides for a fine of up to 1 million riel (?) for vendors who do. Enforcement of this law is weak. MoH has right to prosecute but prosecution through the legal system is seldom successful.