Disease name
advertisement
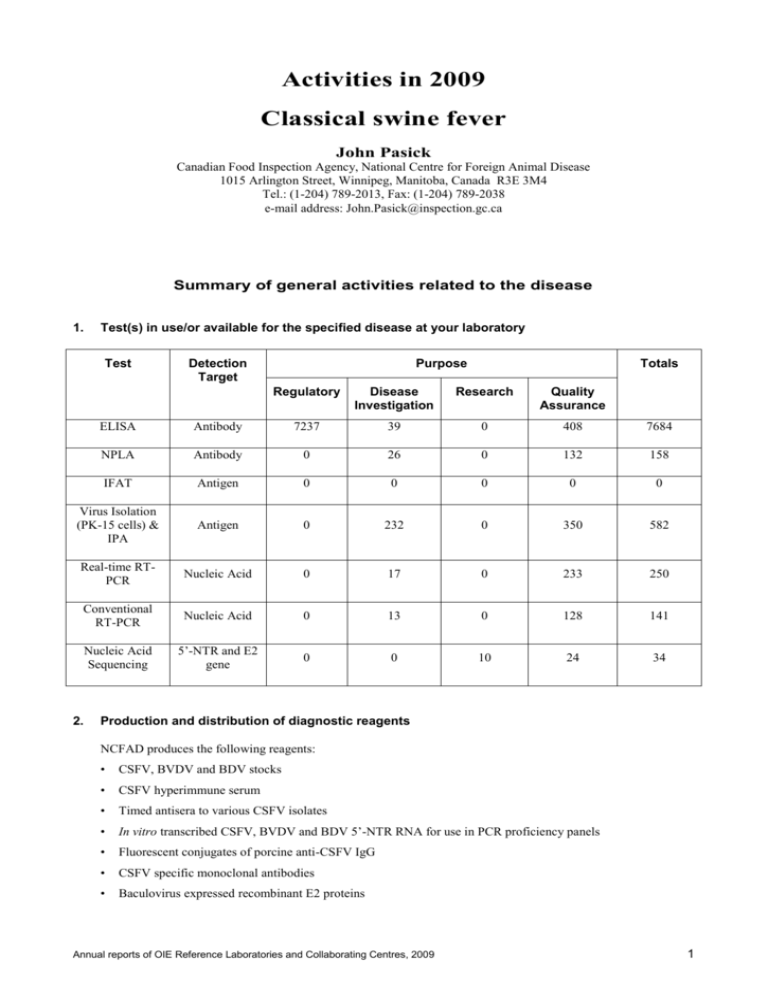
Activities in 2009 Classical swine fever John Pasick Canadian Food Inspection Agency, National Centre for Foreign Animal Disease 1015 Arlington Street, Winnipeg, Manitoba, Canada R3E 3M4 Tel.: (1-204) 789-2013, Fax: (1-204) 789-2038 e-mail address: John.Pasick@inspection.gc.ca Summary of general activities related to the disease 1. Test(s) in use/or available for the specified disease at your laboratory Test Detection Target Purpose Totals Regulatory Disease Investigation Research Quality Assurance ELISA Antibody 7237 39 0 408 7684 NPLA Antibody 0 26 0 132 158 IFAT Antigen 0 0 0 0 0 Virus Isolation (PK-15 cells) & IPA Antigen 0 232 0 350 582 Real-time RTPCR Nucleic Acid 0 17 0 233 250 Conventional RT-PCR Nucleic Acid 0 13 0 128 141 Nucleic Acid Sequencing 5’-NTR and E2 gene 0 0 10 24 34 2. Production and distribution of diagnostic reagents NCFAD produces the following reagents: • CSFV, BVDV and BDV stocks • CSFV hyperimmune serum • Timed antisera to various CSFV isolates • In vitro transcribed CSFV, BVDV and BDV 5’-NTR RNA for use in PCR proficiency panels • Fluorescent conjugates of porcine anti-CSFV IgG • CSFV specific monoclonal antibodies • Baculovirus expressed recombinant E2 proteins Annual reports of OIE Reference Laboratories and Collaborating Centres, 2009 1 Classical swine fever The following amounts were supplied nationally and for use by our own laboratory • 145 real-time CSF RT-PCR proficiency panels NCFAD has not supplied any reagents to OIE member countries in 2009. Activities specifically related to the mandate of OIE Reference Laboratories 3. International harmonisation and standardisation of methods for diagnostic testing or the production and testing of vaccines NCFAD participates in the Inter-Laboratory Comparison Test organized by the EU Reference Laboratory for Classical Swine Fever in Hannover, Germany 4. Preparation and supply of international reference standards for diagnostic tests or vaccines None. 5. Research and development of new procedures for diagnosis and control Work continues on the development of serological tests for CSF using CSFV-specific monoclonal antibodies and recombinant E2 proteins that have been produced in house. 6. Collection, analysis and dissemination of epizootiological data relevant to international disease control None. 7. Provision of consultant expertise to OIE or to OIE Members None. 8. Provision of scientific and technical training to personnel from other OIE Members One scientist from the Estados Unidos para la Prevención de la Fiebre Aftosa y Otras Enfermedades Exóticas de los Animales, Mexico was trained in CSF diagnostic tests that included the neutralisation peroxidase-linked assay, virus isolation, immunoperoxidase assay, conventional RT-PCR and real-time RT-PCR. 9. Provision of diagnostic testing facilities to other OIE Members None. 10. Organisation of international scientific meetings on behalf of OIE or other international bodies None. 11. Participation in international scientific collaborative studies A collaborative study with the Laboratory of Virology, Faculty of Veterinary Medicine, San Marcos University, Peru was carried out to molecularly characterize CSF viruses that were isolated from domestic pigs from different regions of Peru in 2008. All virus isolates were found to belong to genetic sub-group 1.1 consistent with the sub- 2 Annual reports of OIE Reference Laboratories and Collaborating Centres, 2009 Classical swine fever group of viruses that have been identified from other South American countries. Although the Peruvian isolates are most closely related to viruses from Colombia and Brazil, they form a monophyletic clade, which suggests they have a distinct evolutionary history. 12. Publication and dissemination of information relevant to the work of OIE (including list of scientific publications, internet publishing activities, presentations at international conferences) Presentations at international conferences and meetings None. Scientific publications in peer-reviewed journals None. Other communications None. 13. Inscription of diagnostic kits on the OIE Register i) Did you participate in expert panels for the validation of candidate kits for inscription on the OIE Register? If yes, for which kits? No. ii) Did you submit to the OIE candidate kits for inscription on the OIE Register? If yes, for which kits? No. _______________ Annual reports of OIE Reference Laboratories and Collaborating Centres, 2009 3