Trade Barriers Working Group Meeting by Teleconference 24 June
advertisement

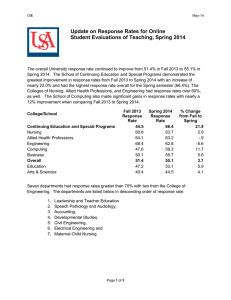
Trade Barriers Working Group Meeting by Teleconference 24 June 2013 Meeting notes 1. International Pet Food Safety Standard (referred to Safety WG)- FEDIAF confirmed that this WG is assembling responses and a meeting is planned shortly. 2. Seek endorsement of key regulators (for Safety Standard). Potential for an alliance with OIE (similar to World Renderers Organisation as attached). Brief background provided following earlier information circulated concerning World Renderers Organisation and their OIE co-operation agreement. Discussion included comments on need for consistent approaches amongst GAPFA members, suggestion to commence discussion at local level to build relationships and understanding, concern that OIE guidelines are differently interpreted leading to potential barriers and query if the WG might seek different interpretations for petfood having a different risk profile to the food chain. Agreed that PFIAA would seek feedback from ARA-WRO on their experience with the co-operation agreement thus far. 3. Labelling and claims- compile a spread sheet which compares requirements for different markets. (Target was defined as to achieve a best practice label template) Noted additions for US and EU just received and now added to the spreadsheet. Members to consider common elements for possible incorporation into a “best practice” international guideline document. Members noted that there are different legal and guideline requirements for different jurisdictions. Also noted that inclusion of other markets would be helpful particularly in Asia and South America and members to seek inputs form these regions for inclusion. Members discussed variable approaches to therapeutics and potential trade barriers which may result. An example from Japan of additional duty payable if protein exceeds 35% was tabled. Other examples are welcomed from members to better understand how the differing approaches might impact on trade. PFIAA raised issue of “low risk” nutritional Petfoods being treated differently to (higher risk) therapeutic veterinary medicines and the advantages of approaches such as PARNUTS listing of “accepted nutrition-health relationships” from EU. 4. Establish key contacts- a register of key agency/government contacts (for GAPFA member reference) An initial spreadsheet has been partly populated but needs further data. The spreadsheet to be recirculated for WG member inputs. 5. Consider a review of tariff levels and impacts- prioritise markets for investigation. Little progress on this item thus far. Japan raised tariff on >35% protein foods. Members asked to submit examples for further discussion. 6. Next meeting and target outcomes Follow up items Safety WG inputs on potential trade barriers OIE - Discuss safety and OIE relationships at local levels and feedback to WG o - Ask WRO for their experiences on OIE co-operation agreement – PFIAA - Labelling & Claims: - Consider common elements in labelling spreadsheet – Spreadsheet to be circulatedSeek input from other markets, eg Asia. Sth America – PFIAA & Members to contact - Comment and consider if a low risk petfood approach to therapeutics such as PARNUTS should be a potential WG goal Key Regulatory Contacts - Populate further the key contacts spreadsheet. - Members Tariff Barriers - Seek views and examples on tariff barriers to trade for discussion - Members Next meeting Tuesday 20 August Midday GMT (13 hours ahead of this June meeting time)