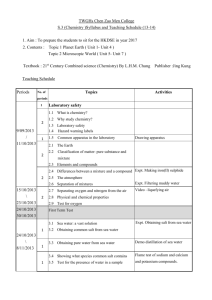
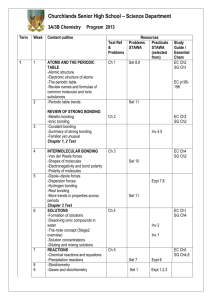
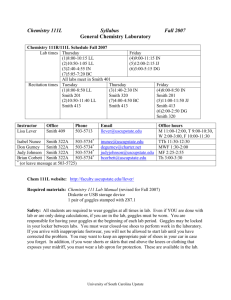
List of Laboratory Experiments Chemistry 401G – Inorganic
advertisement
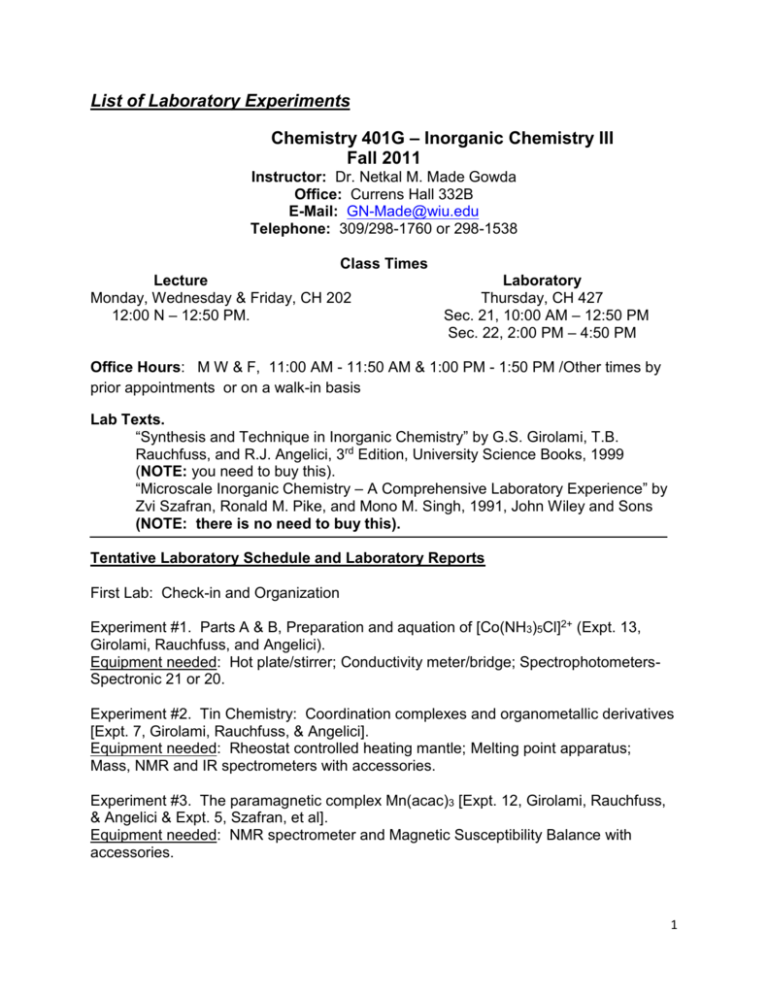
List of Laboratory Experiments Chemistry 401G – Inorganic Chemistry III Fall 2011 Instructor: Dr. Netkal M. Made Gowda Office: Currens Hall 332B E-Mail: GN-Made@wiu.edu Telephone: 309/298-1760 or 298-1538 Class Times Lecture Monday, Wednesday & Friday, CH 202 12:00 N – 12:50 PM. Laboratory Thursday, CH 427 Sec. 21, 10:00 AM – 12:50 PM Sec. 22, 2:00 PM – 4:50 PM Office Hours: M W & F, 11:00 AM - 11:50 AM & 1:00 PM - 1:50 PM /Other times by prior appointments or on a walk-in basis Lab Texts. “Synthesis and Technique in Inorganic Chemistry” by G.S. Girolami, T.B. Rauchfuss, and R.J. Angelici, 3rd Edition, University Science Books, 1999 (NOTE: you need to buy this). “Microscale Inorganic Chemistry – A Comprehensive Laboratory Experience” by Zvi Szafran, Ronald M. Pike, and Mono M. Singh, 1991, John Wiley and Sons (NOTE: there is no need to buy this). Tentative Laboratory Schedule and Laboratory Reports First Lab: Check-in and Organization Experiment #1. Parts A & B, Preparation and aquation of [Co(NH3)5Cl]2+ (Expt. 13, Girolami, Rauchfuss, and Angelici). Equipment needed: Hot plate/stirrer; Conductivity meter/bridge; SpectrophotometersSpectronic 21 or 20. Experiment #2. Tin Chemistry: Coordination complexes and organometallic derivatives [Expt. 7, Girolami, Rauchfuss, & Angelici]. Equipment needed: Rheostat controlled heating mantle; Melting point apparatus; Mass, NMR and IR spectrometers with accessories. Experiment #3. The paramagnetic complex Mn(acac)3 [Expt. 12, Girolami, Rauchfuss, & Angelici & Expt. 5, Szafran, et al]. Equipment needed: NMR spectrometer and Magnetic Susceptibility Balance with accessories. 1 Experiment #4. Optical resolution of [Co(en)3]3+ [Handout]. Equipment needed: Hot plates/stirrers; Polarimeter with accessories. Experiment #5. The Metal-Arene Complex [1,3,5-C6H3(CH3)3]Mo(CO)3 [Expt. 16, Girolami, Rauchfuss, & Angelici]. Equipment needed: Rheostat controlled heating mantles; IR spectrometers. Experiment #6. Synthesis of metal complexes of DMSO Part A: Preparation of CuCl2.2DMSO Part B: Preparation of PdCl2.2DMSO (Expt. 20, Microscale, Szafran et al.) Equipment needed: Melting point apparatus; Hot plates/stirrers; IR spectrometers. Experiment #7. Synthesis of metal acetylacetonates Part A: Preparation of Tris (2,4-pentanedionato)chromium(III) (Expt. 22, Microscale, Szafran et al.) Equipment needed: Melting point apparatus; Hot plates/stirrers; IR spectrometers. NOTE: Some experiments will be performed at the microscale level. Other experiments will be added, if time permits. There will be pre-lab quizzes (5-10 min.) on some/all experiments. Lab Reports Each lab report should be 4-10 pages in length. To prepare lab reports, you should use the following American Chemical Society’s format. 1. Introduction: A brief background information and theory concerning the experiment should be presented here. 2. Experimental or Materials & Methods: A list of the reagents and equipment, and a brief description of the procedures used should be presented here. 3. Results and Discussion: This section should involve presentation as well as a brief discussion of your experimental data. 4. Conclusion: This includes the following: summary statements which reflect insights you have gained and the statements concerning your attempt to analyze the likely kinds of experimental errors affecting your results. 5. References: List literature references quoted in the text. 2 The lab reports should be submitted by 12:00 noon on the Wednesday following completion of the experiment, unless otherwise directed. NOTE: Points will be deducted from the score for late submission and if graphs and spectra with appropriate labeling are not included in the lab report. In the revision of lab reports for WID credit, original experimental data obtained by the student should not be changed. WID Standards to be Fulfilled by Majors: The instructor reads and makes written comments on the first draft of each report (restricted to first two experiments). The instructor also encourages peer review of the draft before submitting the final report for grading. The second/final draft will be graded. The forensic chemistry majors will have conferences with the forensic faculty member and get the training needed to prepare forensic/criminal investigation reports to be submitted to criminal/forensic labs or courts. Moreover, they get additional exposure to the standard writing format of criminal lab reports in their 400-level Forensic Chemistry lab classes. 3