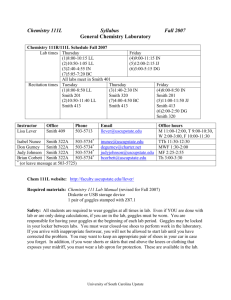
Teaching schedule
advertisement
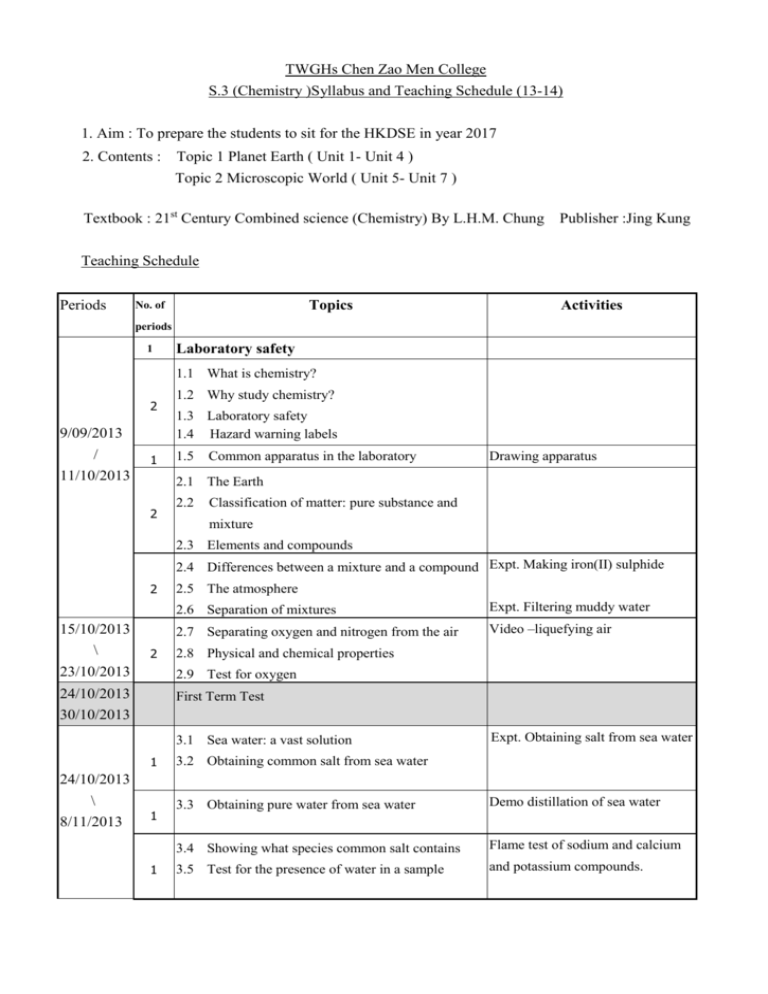
TWGHs Chen Zao Men College S.3 (Chemistry )Syllabus and Teaching Schedule (13-14) 1. Aim : To prepare the students to sit for the HKDSE in year 2017 2. Contents : Topic 1 Planet Earth ( Unit 1- Unit 4 ) Topic 2 Microscopic World ( Unit 5- Unit 7 ) Textbook : 21st Century Combined science (Chemistry) By L.H.M. Chung Publisher :Jing Kung Teaching Schedule Periods Topics No. of Activities periods 1 Laboratory safety 1.1 What is chemistry? 2 9/09/2013 / 11/10/2013 1 1.2 Why study chemistry? 1.3 Laboratory safety 1.4 Hazard warning labels 1.5 Common apparatus in the laboratory Drawing apparatus 2.1 The Earth 2 2.2 Classification of matter: pure substance and mixture 2.3 Elements and compounds 2.4 Differences between a mixture and a compound Expt. Making iron(II) sulphide 2 15/10/2013 \ 23/10/2013 2 2.5 The atmosphere 2.6 Separation of mixtures Expt. Filtering muddy water 2.7 Separating oxygen and nitrogen from the air Video –liquefying air 2.8 Physical and chemical properties 2.9 Test for oxygen 24/10/2013 30/10/2013 First Term Test 3.1 Sea water: a vast solution 1 24/10/2013 \ 8/11/2013 1 1 Expt. Obtaining salt from sea water 3.2 Obtaining common salt from sea water 3.3 Obtaining pure water from sea water Demo distillation of sea water 3.4 Showing what species common salt contains Flame test of sodium and calcium 3.5 Test for the presence of water in a sample and potassium compounds. 3.6 Electrolysis of sea water 11/11/2013 Expt. Electrolysis of sea water 3.7 Uses of the products obtained by the electrolysis \ 15/11/2013 2 of sea water 3.8 The particle theory of matter 3.9 Physical and chemical changes 18/11/2013 \ 28/11/2013 2 4.1 Metals in the Earth’s crust Expt. Reduction of lead oxide and 4.2 Extracting metals from their ores copper (II) oxide 4.3 Investigating calcium carbonate converting egg shell into lime water. 2 4.4 Formation of chalk, limestone and marble 4.5 Formation of limestone caves 5.1 What is an element made of ? 2 Demo: Inspection of some elements 5.2 Symbols for elements 5.3 states of elements 5.4 How to classify elements 2/12/2013 \ 20/12/2013 2 1.5 1.5 5.5 Basic structure of an atom 5.6 Atomic number and mass number 5.8 Isotopes 5.9 Relative masses of atoms and the carbon-12 scale Ex. Electronic configurations Ex. Calculation on relative 5.10 The arrangement of electrons in atoms 5.11 Electrons and orbitals 23/12/2013 1/1/2014 Christmas holiday 2/1/2014 14/1/2014 1st Term Examination 15/1/2014 2 6.1 How to group elements together ? abundance of elements The periodic table game 23/1/2014 27/1/2014 6/2/2014 7/2/2014 20/2/2014 24/02/2014 \ 25/3/2014 Lunar New Year Holiday 2 6.2 The periodic table 2 6.3 Patterns across the periodic table 2 2 2 2 6.4 Group 1 elements to group 0 elements 6.8 Predicting the chemical properties 6.9 From atoms to ions and predicting the charge 7.1 Conductors ,electrolytes and non-conductors 7.2 Evidence of ions Revision Expt. Migration of colour ions 26/3/2014 1/4/2014 2/4/2014 17/4/2014 Second term test 2 7.3 Chemicals bonds 2 7.4 Names of 18/4/2014 26/4/2014 28/4/2014 23/5/2014 ions and colour of compounds Easter Holiday 4 7.5 chemical formulae of ionic compounds Investigating model of giant ionic 7.6 Metallic bonds structure 2 7.7 Naming ionic compounds 26/5/2014 \ 2 7.8 Colours of ionic compounds 2 7.9 Chemical formulae of ionic compounds 6/6/2014 2 7.10 Metallic bonds in metals 10/6/2014 \ 20/6/2014 Final Examination Play the card game