640
advertisement

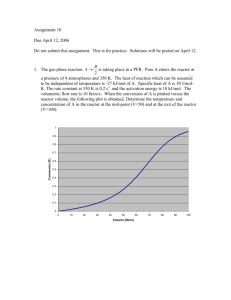
Chapter 4 The irreversible gas phase reaction A+B2C is carried out in a PFR reactor. The reaction is non-elementary in that it is first order in A and zero order in B. The feed is equal molar in A and B, and the pressure at the entrance is 10atm and the pressure at the exit is 2atm. Additional information: FA0=100 mol/s K=1dm3/kg.s CA0=1mol/ dm3 1) What is the conversion exiting the reactor? 2) If the flow were turbulent, what would be pressure drop if the cross sectional area is increased by a factor 2 and the particle size decreased by a factor of 10? All other conditions remain the same. (Hint: in the turbulent flow, the Ergun equation neglects the part of 150(1-) /Dp. Reference: R.B.Bird, transport phenomena (New York, Wiley, 1960), p. 200.) Answer: 1) X=0.5 2) Pressure drops too great, no flow under these conditions. This problem is to study the pressure drop in PBR. Mole balance, rate law, stoichiometry and Ergun equation are used to solve the problem. The main point: 1. Calculate using the pressure drop 2. The relation between 0 and cross sectional area and particle diameter. Solution: dx ra' 1) dw FA0 ra' kCA C A C A0 (1 x) p p0 dx KC A0 (1 x) p dw FA0 p0 p 2 0 (1 W ) 0.5 (1 W ) 0.5 0.0096 Kg 1 p0 10 dx KC A0 (1 x) (1 W ) 0.5 dw FA0 KC A0 1 1 0.01 FA0 100 KC A0 dx (1 W ) 0.5 dW 1 x FA0 KC A0 dx 0 1 x FA0 x 2) 0 (1 W ) 0.5 dW x 0.5 2 0 Ac (1 ) c p 0 0 0 Ac So 100 G2 Dp (For turbulent flow) 1 A Dp 3 c Ac , 2 2 Ac ,1 D p,2 1 D p ,1 10 1 Ac , 2 D p , 2 1 3 8 0.8 2 1.25 1 2 Ac ,1 D p ,1 10 3 Calculate the new pressure drop p (1 2W ) 0.5 (1 1.25 0.0096 100) 0.5 (0.2) 0.5 p0 Before the exit of PFR, p=0 G 1 Ac Chapter 8 The elementary reversible gas phase reaction A 2B is to be carried out adiabatically. Pure A is fed at a rate of 2 mol/min, at temperature of 373 K, and at concentration of 0.01 mol/dm3. Additional information: CPA 5.0cal /( mole K ) CPB 2.5cal /( mole K ) HR= -3500 cal/mol at 373 K Ke=0.05 at 373 K What is the adiabatic equilibrium temperature and conversion? Answer: xe 0.45 T 688K This problem is to study the steady-state nonisothermal reactor. Stoichiometry, the relation between equilibrium constant and conversion, Van’t Hoff’s equation and energy balance are used to solve problem The main point: mole balance and energy balance. Solution: Mole balance: =1 1 x T0 C A C A0 ( ) 1 x T Ke CB 2C A0 x T0 1 x T 4C A2 0 xe2T02 (1 xe )T K eT xe2 C B2 C A (1 xe ) 2 TC A0 (1 xe )T 4C A0T0 1 xe2 K eT 4C A0T0 xe K eT 1 4C A0T0 4C A0T0 4 0.01 373 14.92 1 1 1 T 373 3500 1 0 K e K e (T1 ) exp H Rx ( 0.05 exp ( ) 0.05 exp( 4.722 ) T1 T ) T 1.987 373 T Energy balance: CP=0 x EB C i pi (T T0 ) H Rx (T ) 5(T 373) 3500 T 373 )T T xe T 373 14.92 0.05 exp( 4.722 )T T 0.05 exp( 4.722 Combining the last two equations: x EB xe xe 0.45 T 688K Chapter 12 Consider a gas-solid (catalyst) reaction A( g ) B( g ) in which the reaction is zero-order, and the solid particles have “slab” or “flat-plat” geometry with two faces permeable to A. The thickness is 2L. 1) Derive the diffusion equation in nondimensional form for A together with the expression for the Thiele Modulus. 2) Solve the equation in 1) to give the nondimensional concentration Answer: d 2 1) 02 0 2 d 1 2) 02 (2 1) 1 2 This problem is to study the Thiele Modulus in rectangular coordinate and to solve concentration profile with boundary conditions. Shell balance, rate law, Fick’s law, dimensionless and boundary theory are used to solve the problem The main point: 1. Shell balance 2. The second boundary is hard to get Solution: 1) S is the cross-section area for catalyst. S WAZ Z S WAZ WAZ De Z Z dC A dZ rA' c SZ 0 rA' rA" S a K 0 S a (For zero order) Substitute the above two equations into the first one and divided by SZ d 2C A K 0 c S a 0 De dZ 2 Let CA C As Z L d 2 C A d 2 C As 2 2 dZ 2 d L K0 c Sa Let 0 L De C As d 2 L2 K 0 c S a 0 De C As d2 d 2 02 0 2 d 2) Solve the diffusion equation: 1 2 02 2 c1 c 2 BC: At the surface c1, c2 are constants Z L 1 L L At the center 0 d d 0 02 0 d 0 d c1 0 c1 0 1 1 From first BC: 1 02 c 2 c 2 1 02 2 2 1 So 02 (2 1) 1 2 C A C As 1 C As C As (1) (2) Chapter 13 The following concentration readings are of response to an impulse input of tracer T(min) 0 C(mol/L) 0 10 1 20 3 30 5 40 5 50 4 60 2 70 1 80 0 What conversion (using zero-parameter model) can be expected in this reactor of a first order reaction that has a conversion of 82.18% in an ideal CSTR at the same residence time? Answer: 0.962 This problem is to study distribution of residence time Residence-time distribution function, mean residence time, rate law and zero-parameter model are used to solve the problem. The main point is to calculate mean residence time and reaction constant. Solution: Using polymath 80 0 E (t ) CdT 211.5 C (t ) 80 0 t C 0 10 20 30 40 50 60 70 80 0 1 3 5 5 4 2 1 0 C (t )dt E(t) tE(t) 0 0.004728 0.014184 0.023641 0.023641 0.018913 0.009456 0.004728 0 0 0.047281 0.283688 0.70922 0.945626 0.945626 0.567376 0.330969 0 80 t m tE(t )dt 38.62 0 First order reaction in an ideal CSTR: X k 1 k 0.8218 k 38.62 k 0.1194 1 k 38.62 Segregation conversion of first order reaction is given by: 80 X 1 e kt E (t )dt 1 0.038 0.962 0 t exp(-kt) E(t) exp(-kt)E(t) 0 10 20 30 40 50 60 70 80 1 0.30301 0.09181 0.02782 0.00843 0.00255 0.00077 0.00023 7.11E-05 0 0.00473 0.01418 0.02364 0.02364 0.01891 0.00946 0.00473 0 0 0.00143 0.0013 0.00066 0.0002 4.83E-05 7.32E-06 1.11E-06 0 tm