RR5Does the amount of the catalyst manganese (IV) oxide change
advertisement

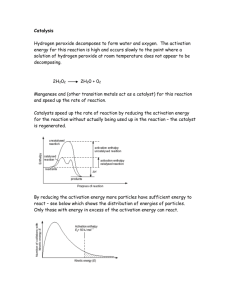
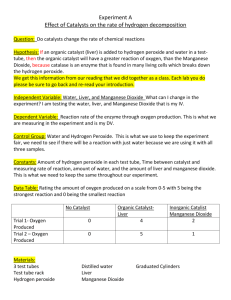
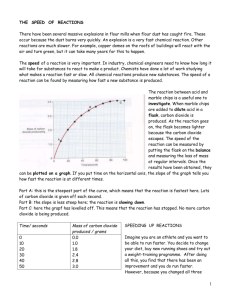
RR5:Does the amount of the catalyst manganese (IV) oxide change during the decomposition of hydrogen peroxide? IDEA Oxygen is given off when hydrogen peroxide decomposes. Each student group starts off with the same amount of hydrogen peroxide, but with different amounts of manganese (IV) oxide (MnO2) How much manganese dioxide MnO2 is used up, if any during the course of the chemical reaction ? WORD EQUATION manganese dioxide catalyst hydrogen peroxide CHEMICAL EQUATION manganese dioxide catalyst H2O2 APPARATUS water O2 + + oxygen H2O PROCEDURE 1. Put a 1-2 spatulas of black manganese dioxide into the evaporating basin. 2. 3. Place the evaporating basin on a tripod. 4. Now SLOWLY add 5-10 mls of hydrogen peroxide onto the manganese dioxide. 5. A chemical reaction occurs. Record its mass. 6. The breakdown of hydrogen peroxide is catalysed by the black manganese dioxide. 7. Heat the contents of the evaporating basin gently until all the products have been removed DO NOT LET IT SPIT 8. Let the evaporating basin cool down. 9. Reweigh the evaporating basin and contents. 10. Mass before heating = ...........................g 11. Mass after heating = ..............................g CONCLUSION In this experiment how did the mass of the catalyst before and after the experiment change ? ................................................................................................................................................................ ................................................................................................................................................................ FOR THE TECHNICIAN HAZARDS Electronic balance 20 vol hydrogen peroxide Tripod Corrosive – may cause burns Bunsen Burner Dangerous if swallowed evaporating basin or tin lid manganese dioxide powder Manganese dioxide 20 vol hydrogen peroxide Harmful if breathed in or swallowed. small measuring cylinder