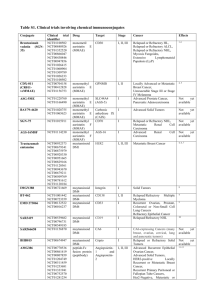
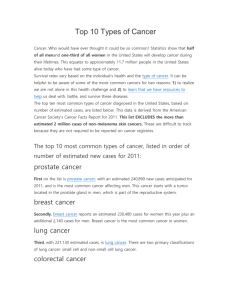
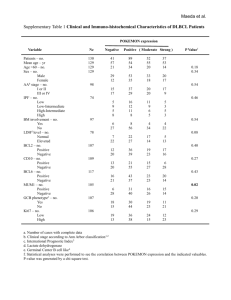
Table S2. Clinical trials involving radioimmunoconjugates
advertisement

Table S2. Clinical trials involving radioimmunoconjugates Conjugate Clinical trial identifier 63 clinical trials in clinicaltrials.gov Drug Target Stage Cancer Effects 90Yttrium CD20 I, II, III, IV Follicular lymphoma, Relapsed And Refractory NHL, Relapsed And Refractory MCL, DLBCL, CNS Lymphoma, Low Grade NHL, Multiple Myeloma, Waldenstrom's Macroglobulinemia 1-4 NCT01101581 NCT01147393 NCT01354457 90Yttrium CD22 I, II Relapsed/Refractory, Aggressive NHL, Follicular Lymphoma , Relapsed or Refractory CD22+ B-ALL 5-7 90Yttrium Tac I, II Cutaneous CTCL, ATL 8 Anti-Tac 90YttriumLabetuzumab NCT00001249 NCT00019227 NCT00040599 NCT00041652 NCT00041639 90 Carcinoemb ryonic antigen (CEA) I, II Refractory Metastatic/Recurrent Colorectal Carcinomas Recurrent, Metastatic, Unresectable Colorectal and Breast Carcinomas Refractory Advanced/Metastatic Pancreatic Carcinoma Not yet available 90Yttrium- NCT00072410 90Yttrium Lewis Y I Advanced Ovarian Cancer Not yet available hu3S193 90YttriumDOTA-cG250 NCT00199875 90Yttrium I Advanced Renal Cancer Not yet available 131Iodine I, II, III 131Iodine- NCT00291486 131Iodine A33 I huA33 131Iodine-CC49 NCT00025532 NCT00023933 131Iodine TAG-72 I Relapsed And Refractory NHL, HL, Multiple Myeloma, Mantle Cell Lymphoma, Metastatic Colorectal Cancer Gastrointestinal Adenocarcinoma, Colon Cancer 9-11 Tositumomab 38 clinical trials in clinicaltrials.gov Carbonic anhydrase IX (CA IX) CD20 131Iodine- NCT00003102 131Iodine I, II Advanced Renal Cancer 14, 15 NCT01296204 NCT00199888 131Iodine I II Multiple Myeloma Refractory Metastatic Renal Cell Carcinoma Not yet available NCT00199862 124Iodine carbonic anhydrase IX (CA IX) CD138 Carbonic anhydrase IX (CA IX) A33 I Colorectal Cancer Not yet available NCT00916123 NCT00538668 NCT00859781 NCT00195039 NCT00967577 NCT00081172 177Lutetium Prostate specific membrane antigen (PSMA) Pilot study, II Metastatic AndrogenIndependent Prostate Cancer, Relapsed Prostate Cancer, Nonprostate Metastatic Solid Tumors 18, 19 90Yttrium- Ibritumomab tiuxetan 90Yttrium- Epratuzumab 90Yttrium- 131Iodine- Yttrium cG250 131Iodine-BB4 124Iodine- 124Iodine cG250 124IodinehuA33 177LutetiumJ591 12 13 16, 17 ALL = Acute Lymphoblastic Leukaemia; ATL = Adult T-Cell Leukaemia; CTCL = Cutaneous TCell Lymphoma; DLBCL = Diffuse Large B-Cell Lymphoma; HL = Hodgkin’s Lymphoma; MCL = Mantle Cell Lymphoma; NHL = Non-Hodgkin’s Lymphoma References 1. Han EJ, Lee SE, Kim SH, et al. Clinical outcomes of post-remission therapy using (90)yttrium ibritumomab tiuxetan (Zevalin(R)) for high-risk patients with diffuse large B-cell lymphoma. Ann Hematol 2011. 2. Vitolo U, Barosi G, Fanti S, et al. Consensus conference on the use of 90-yttrium-ibritumomab tiuxetan therapy in clinical practice. A project of the Italian society of hematology. Am J Hematol 2010;85:147-55. 3. Jacobs SA. Yttrium ibritumomab tiuxetan in the treatment of non-Hodgkin's lymphoma: current status and future prospects. Biologics 2007;1:215-27. 4. Zhou Y, Zhang L, Romaguera J, et al. Immunotherapy in mantle cell lymphoma: anti-CD20-based therapy and beyond. Am J Hematol 2008;83:144-9. 5. Morschhauser F, Kraeber-Bodere F, Wegener WA, et al. High rates of durable responses with antiCD22 fractionated radioimmunotherapy: results of a multicenter, phase I/II study in non-Hodgkin's lymphoma. J Clin Oncol 2010;28:3709-16. 6. Bodet-Milin C, Kraeber-Bodere F, Dupas B, et al. Evaluation of response to fractionated radioimmunotherapy with 90Y-epratuzumab in non-Hodgkin's lymphoma by 18F-fluorodeoxyglucose positron emission tomography. Haematologica 2008;93:390-7. 7. Linden O, Hindorf C, Cavallin-Stahl E, et al. Dose-fractionated radioimmunotherapy in nonHodgkin's lymphoma using DOTA-conjugated, 90Y-radiolabeled, humanized anti-CD22 monoclonal antibody, epratuzumab. Clin Cancer Res 2005;11:5215-22. 8. Waldmann TA, White JD, Carrasquillo JA, et al. Radioimmunotherapy of interleukin-2R alphaexpressing adult T-cell leukemia with Yttrium-90-labeled anti-Tac. Blood 1995;86:4063-75. 9. Watanabe T. Treatment strategies for nodal and gastrointestinal follicular lymphoma: current status and future development. World J Gastroenterol 2010;16:5543-54. 10. Park SI, Press OW. Radioimmunotherapy for treatment of B-cell lymphomas and other hematologic malignancies. Curr Opin Hematol 2007;14:632-8. 11. Leahy MF, Turner JH. Radioimmunotherapy of relapsed indolent non-Hodgkin lymphoma with 131I-rituximab in routine clinical practice: 10-year single-institution experience of 142 consecutive patients. Blood 2011;117:45-52. 12. Chong G, Lee FT, Hopkins W, et al. Phase I trial of 131I-huA33 in patients with advanced colorectal carcinoma. Clin Cancer Res 2005;11:4818-26. 13. Murray JL, Macey DJ, Kasi LP, et al. Phase II radioimmunotherapy trial with 131I-CC49 in colorectal cancer. Cancer 1994;73:1057-66. 14. Brouwers AH, Mulders PF, de Mulder PH, et al. Lack of efficacy of two consecutive treatments of radioimmunotherapy with 131I-cG250 in patients with metastasized clear cell renal cell carcinoma. J Clin Oncol 2005;23:6540-8. 15. Divgi CR, O'Donoghue JA, Welt S, et al. Phase I clinical trial with fractionated radioimmunotherapy using 131I-labeled chimeric G250 in metastatic renal cancer. J Nucl Med 2004;45:1412-21. 16. Divgi CR, Pandit-Taskar N, Jungbluth AA, et al. Preoperative characterisation of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-cG250) and PET in patients with renal masses: a phase I trial. Lancet Oncol 2007;8:304-10. 17. Rice SL, Roney CA, Daumar P, et al. The next generation of positron emission tomography radiopharmaceuticals in oncology. Semin Nucl Med 2011;41:265-82. 18. Bander NH, Trabulsi EJ, Kostakoglu L, et al. Targeting metastatic prostate cancer with radiolabeled monoclonal antibody J591 to the extracellular domain of prostate specific membrane antigen. J Urol 2003;170:1717-21. 19. Nakajima T, Mitsunaga M, Bander NH, et al. Targeted, Activatable, In Vivo Fluorescence Imaging of Prostate-specific Membrane Antigen (PSMA)-positive Tumors Using the Quenched Humanized J591 Antibody-ICG Conjugate. Bioconjug Chem 2011. Table S2: Details of the clinical trials involving radioimmunoconjugates (data gathered from US clinicaltrials.gov, Clinical Trials register EU and available bibliography in sponsor an PubMed websites).