Chemistry Fundamentals Worksheet
advertisement
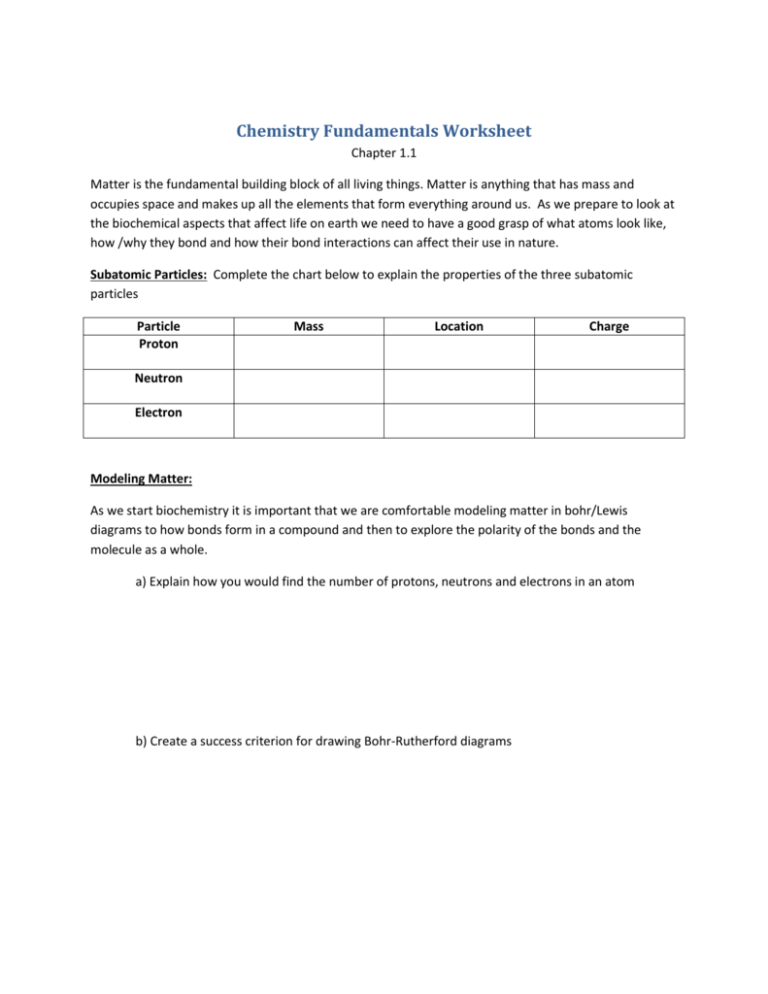
Chemistry Fundamentals Worksheet Chapter 1.1 Matter is the fundamental building block of all living things. Matter is anything that has mass and occupies space and makes up all the elements that form everything around us. As we prepare to look at the biochemical aspects that affect life on earth we need to have a good grasp of what atoms look like, how /why they bond and how their bond interactions can affect their use in nature. Subatomic Particles: Complete the chart below to explain the properties of the three subatomic particles Particle Proton Mass Location Charge Neutron Electron Modeling Matter: As we start biochemistry it is important that we are comfortable modeling matter in bohr/Lewis diagrams to how bonds form in a compound and then to explore the polarity of the bonds and the molecule as a whole. a) Explain how you would find the number of protons, neutrons and electrons in an atom b) Create a success criterion for drawing Bohr-Rutherford diagrams c) Summarize the reasons why ions form and how we show ions d) Draw Lewis diagrams for Chlorine, Boron, Magnesium ion and oxide ion Isotopes: Recall that the two parts of the atom that give it mass are the protons and the neutrons in the nucleus. Each of these particles have a mass of 1amu, but you will notice as you look at the atomic mass of atoms on the periodic table that they don’t have a whole number for their atomic mass. a) What is an isotope? b) How do isotopes affect the atomic mass of the element? c) Summarize how radioisotopes can be useful: Chemical Bonding Electron configuration around an atom, or within the bonds of a compound, is organized and purposeful. As we have explored orbitals in the past, electrons have areas where they are most likely to be found, not as simplistically as in a Bohr diagram, but it still hold. Summarize the shapes of the s, p, d and f orbitals and then explain how many electrons each of these types of orbitals can hold. P Number of electrons S Number of electrons D Number of electrons F Number of electrons Keep in mind that elements form compounds to become stable. Regardless of how many electrons an element has in its valence shell it will work to become stable by either gaining/losing electrons or sharing electrons until it has no empty spaces in its valence shell. Define: Intermolecular forces of attraction Summarize properties of ionic and covalent bonds in the space below: Electronegativity and Polarity What is electronegativity: What trends do you notice about electronegativity values in the periodic table? How do you determine the electronegative difference of a bond? Which bonds have low electronegative differences and which have high electronegative differences? Define: Hybridization Table 5: pg. 15 Summarize the rules that would help you to determine whether a bond was polar. Create a success criteria you could use to determine if a molecule was polar or non-polar. Water: The Versatile Solvent Water is among the most abundant and most important molecules found on earth. Without water and special biochemical properties that it has life as we know it would cease to exist. a) Define: Intermolecular bonds b) Summarize the intermolecular forces discussed in your text c) Explain how we can determine if a molecule will exert an intermolecular force on a near by molecule or not. d) Explain what is meant by hydrophobic and hydrophilic e) Discuss how the unique properties of water allow life on earth to exist.