Chemical Bonding Review Worksheet: High School Chemistry
advertisement

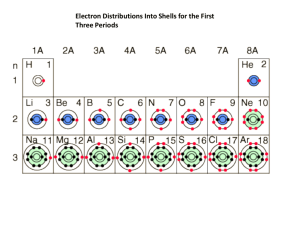
Bonding Review Chem A/B Answer the following questions neatly on a separate sheet of paper. NOTE: 1. “Lewis Structure” means Electron Dot Diagram. 2. Use the Sargent Welch Periodic Table for electronegativity values. 1. 2. 3. When atoms combine by sharing electrons, the bonding is described as . Do metal elements typically exhibit high electronegativities? Compare to ionization energy. Which of the following compounds contains an ionic bond? a. HCl b. CO c. NaCl d. H2O e. ClBr 4. Use electronegativity values to determine which of the following have nonpolar bonds. a. H2S b. HCl c. Br2 d. OF2 e. CS2 5. The number of polar covalent bonds in CCl2F2 is: 6. What kind of intermolecular force exists between water molecules? 7. The electron configuration for the bromide ion is identical to that of which noble gas? 8. How many electrons does a phosphorous atom need to gain in order to form a stable octet? 9. Which of the following has only double bonds? (use Lewis structures to decide) a. H2O b. C2H2 c. CO2 d. HCN e. all 10. How many covalent bonds does carbon almost always form? 11. True (a) or False (b): Carbon dioxide has ionic bonds. 12. How many unpaired electrons are there in a nitrogen atom? 13. How do you account for the fact that normally, chlorine is a gas, bromine is a liquid and iodine is a solid? 14. How is the bond classified if a bonding pair of electrons is unequally shared between two atoms in a molecule? 15. Which one of the following ions “breaks” the octet rule? (does it show noble gas electron config?) a. Fb. Na+ c. Mg2+ d. Cle. Al2+ 16. Which of the following is a nonpolar molecule containing polar bonds? a. H-CN b. O=C=O c. H-Cl d. F-F e. CH2Cl2 17. Draw the Lewis structure for HCN. 18. How many resonance structures are possible to be drawn for the nitrate ion (NO3-)? 19. Draw the electron dot diagram (Lewis structure) for the sulfur atom. 20. Draw the Lewis structure for the Cl2 molecule. 21. Draw the Lewis structure for NI3. Use periodic trends in electronegativity and “delta” ( δ ) notation to show bond polarity in the following bonds. If the bond is nonpolar, write “NP” next to it. Use a table of electronegativity to rank #22-26 in order of increasing bond polarity. 22. F─F 23. H─F 24. Cl─P 25. C─N 26. C─Si 27. Predict whether each of the following is be ionic or covalent: a. MnO2 b. CO c. LiBr d. CrI3, e. SO3 28. Name and describe the intermolecular forces responsible for holding iodine together as a solid. 29. Compare the intermolecular forces in iodine with those that exist in water. 30. Calculate the thickness of a square piece of Al (D=2.70 g/cm3) measuring 0.345 g and 2.50 cm per side. 31. Write a balanced nuclear equation showing the alpha decay of At-209. 32. Write the Complete electron configuration of Tin. How many valence electrons does tin have? 33. Convert 266 km/hr to m/s. 34. Convert 7.13 g/cm3 to kg/m3. 35. Which experiment and who was the experimenter that indicated the presence of the nucleus? What other conclusion was drawn from this experiment?