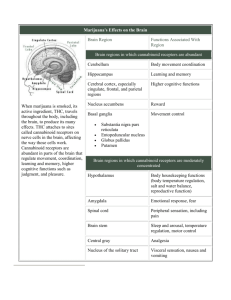
Abstract
advertisement

Department of Chemistry & Biochemistry Seminar Friday, April 15th, 2011 Characterization of GPR35 and GPR55 – Putative Cannabinoid Receptors? Dr. Mary E. Abood Associate Professor of Anatomy and Cell Biology Center for Substance Abuse Research Temple University School of Medicine Host: Dr. Patricia Reggio Two cannabinoid receptor subtypes, CB1 and CB2, have been identified, but the complex pharmacological properties of exogenous cannabinoids and endocannabinoids are not fully explained by their signaling. GPR55 binds a subset of CB1/CB2 ligands and consequently has been proposed as a cannabinoid receptor. This designation, however, is controversial as a result of recent studies, including ones where lysophosphatidylinositol (LPI) is identified as a GPR55 agonist. Using a beta-arrestin green fluorescent protein biosensor as a direct readout of GPR55 activation and phosphorylation of ERK1/2 kinase as a secondary readout we have characterized cannabinoid ligands both as agonists and antagonists. In addition, from a β-arrestin, high-throughput, high-content screen of 300,000 compounds run in collaboration with the Molecular Libraries Probe Production Centers Network initiative (PubChem AID1965 and AID2079), we have now identified potent GPR55 selective agonists and antagonists. Our findings suggest that while GPR55 shares some common ligands with CB1 and CB2, GPR55 selective ligands have been identified and will aid in defining the biological role of this receptor. GPR35 is closely related to GPR55, but turns out to be unresponsive to all cannabinoid ligands that we have tested. In collaboration with Larry Barak and Marc Caron at Duke University, we screened a human GPR35 receptor U2OS cell line (UGPR35β) with the Prestwick Chemical library of 1120 small molecules, 90% being marketed drugs and 10% bioactive alkaloids or related substances. This library was chosen as it presents the greatest possible degree of drug-likeliness. One high potency hit, oxantel pamoate, was obtained from this library. We next tested pyrantel pamoate and pamoic acid (4[(3-carboxy-2-hydroxynaphthalen-1-yl)methyl]-3-hydroxynaphthalene-2-carboxylic acid), also called embonic or pamoic acid, and found that the active compound at GPR35 is pamoic acid, with nanamolar potency. Pamoic acid (as well as zaprinast and kynurenic acid, known agonists at GPR35), induced β-arrestin recruitment, receptor internalization and ERK1/2 activation. Pamoic acid inhibited acetic-acid induced writhing in mice, an in vivo correlate of antinociception, suggesting that GPR35 could be an important drug target. Pamoic acid is used to obtain long-acting formulations of numerous drugs, Our results demonstrate an unexpected biological function for pamoic acid and open potential new uses for a common drug adjuvant. 1:00 p.m. Sullivan Science Building * Wachovia Lecture Hall (Room 201) Refreshments will be served in the lobby immediately following the seminar.








![1-[(tetrahydro-2H-pyran-4-yl)methyl]-1H-indazole-3](http://s3.studylib.net/store/data/007395855_1-e1d40df27c97e9080317a181584a049d-300x300.png)
