Phenacetin Synthesis: Lab Report on 4-Aminophenol Transformation
advertisement

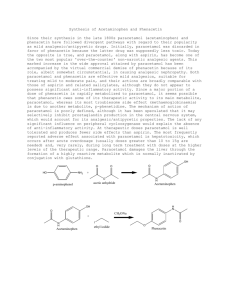
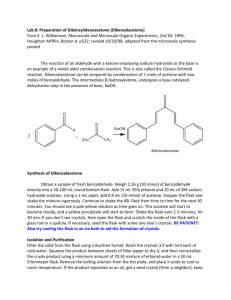
Phenacetin from 4-aminophenol Chemioselective Functional Group Transformations Laboratory report for Chemistry 388 John Stephenson (410-1306) Prepared for Allyson Campbell February 4, 2002 Introduction Phenacetin (4-ethoxyacetanilide) was an over-the-counter pain reliever that was discontinued from the market due to concerns of its nephrotoxic action. Luckily phenacetin’s active metabolite, acetaminophen (4-acetamidophenol), poses little risk of nephrotoxicity and has proven to be a safe, effective pain-killer when used as directed. The hepatoxicity of phenacetin and acetaminophen is attributed to their dangerous oxidation products, acetimidoquinone. To further combine these two pain relievers with alcohol has been shown to be a potent hepatoxic mix. Other popular approved analgesics include ibuprofen, naproxen, ASA (acetylsalicylic acid). Ibuprofen, naproxen and ASA are non-steroidal anti-inflammatory drugs (NSAIDs). Neither acetaminophen nor phenacetin has anti-inflammatory properties, however they are often lumped together and called NSAIDs as well (see commentary). True NSAIDs work by inhibiting cyclo-oxygenase (COX) enzymes necessary in the signaling of pain that would eventually reach the central nervous system (CNS). COX-1 is used in the stomach to produce a lining that protects itself, and sometimes long-term use of non-specific COX inhibitors will cause problems with the stomach lining. This can become so severe that patients will have to discontinue the NSAID. Thankfully much research has gone into developing new COX inhibitors that are specific to COX-2, which is involved in the biosynthesis of prostaglandin that triggers pain and inflammation when tissue is injured. Newer NSAIDs, such as Naproxen and the experimental drug Vioxx, have shown to be more selective to COX-2 inhibition and just as effective with pain management. Before these newer NSAIDs came onto the market, Tylenol and phenacetin remained an alternative choice since they did not pose the same risk of stomach upset. The exact mechanism of phenacetin and acetaminophen’s pain control has not yet been determined, but one theory is that they act in the peripheral nervous system in a way similar to the NSAIDs. In the synthesis outlined in this report, 4-aminophenol will be transformed into phenacetin in a two-step process, with acetaminophen being produced as the intermediate. O O HN NH2 HCl O HN CH3 NaOH O OH CH3 Br O OH OCH2CH3 Results and Discussion 4-acetamidophenol was prepared from 4-aminophenol and acetic anhydride in 76% yield. The resulting product was reacted with ethyl bromide and 4-ethoxyacetanilide formed in 58% yield. Overall, this is a 44% yield of phenacetin from 4-aminophenol. The yield of the first step is satisfactory, typically yields around 85% yield for the conversion from 4-aminophenol to 4-acetamidophenol are seen (Wilbert & De Angelis, 1961). Examples of the formation of phenacetin from acetaminophen are harder to find in literature. Historically, phenacetin was commonly synthesized from 4ethoxyaminophenol so that the route did not infringe on the patents held by WarnerLambert for acetaminophen. However, since our starting material was 4-aminophenol, we needed to form the stable amide bond present in phenacetin before ethoxylating. If the order of this two stage synthesis was reversed and ethyl bromide was reacted with 4-aminophenol in attempts to form 4-ethoxyaminophenol we would see a mixture of N-ethyl substituted analogues as well as substitution on the phenol group. Good chemioselectivity can only be achieved after the amino group is acetylated into an amide bond. The amide bond does not make a good nucleophile and does not react with ethyl bromide. IR spectroscopy showed that acetaminophen did successfully formed from 4aminophenol. The peak at 1654cm-1 is characteristic of amides and would not be present if the 4-aminophenol did not react with acetic anhydride. The peak at 1370cm-1 is characteristic of C-H stretching vibrations present in alkanes and would not be present unless the acetyl group attached to 4-aminophenol. H NMR clearly shows a 2H triplet at 1.374 coupled to a 2H quartet at 3.982 that indicates that the ethyl group replaced then hydrogen atom on the hydroxy group present in acetaminophen to form phenacetin. 1 13 C NMR was not run on this phenacetin sample, however a photocopy of another lab members samples 13C NMR was handed out after the laboratory. The 13C NMR clearly shows the entire structure of Phenacetin. It shows the amide bond formed at 168.753 and the alkoxy group at 63.997. Experimental Procedure Melting points were determined with a Mel-Temp II melting point apparatus made by Laboratory Devices, USA.An Oakton Digital thermometer was used in place of a 400C mercury thermometer. Infrared spectra were recorded with a Bomem Hartmann & Braun MB-series infrared spectrophotometer with the samples prepared on a KBr disk. 1H and 13 C NMR spectra were recorded on a Bruker AC-200 with a sample prepared in CDCl3 and the chemical shift () is reported as downfield from TMS. Part 1: Acetaminophen from 4-Aminophenol Place 4-aminophenol1 (3.0g, 0.028mmol, 1 equiv.) in a 125mL Erlenmeyer flask. To this flask add water (25mL) and conc. HCl (2.3mL). Swirl the flask to dissolve the crystals. Prepare a solution of sodium acetate trihydrate (4.5g) in water (12mL). To the solution of 4-aminophenol in aqueous HCl now add acetic anhydride (3.3g, 3.1mL, 0.033mmol, 1.2 equiv). Swirl the mixture to mix it, add the solution of sodium acetate, swirl again to mix thoroughly then set it aside until crystals start to form. Immersion in cold water then in an ice bath can be carried out after crystals have started to form. Collect the product by suction filtration and wash with a little cold water. Once the crystals have completely dried, determine the yield (3.17g crunchy bright white crystals, 21.0mmol, 76.3%). IR assignments 1654cm-1 836cm-1 1612,1563,1509,1439cm-1 1370cm-1 3324cm-1 3163cm-1 Amide C=O stretching vibrations Aromatic C-H bending vib. (2 adj H atoms) Aromatic C=C stretching vib. Alkane C-H bending vib. Amide N-H stretching vib. Phenolic hydrogen bonding vib. Outline of Experiment - add 3.0g 4-aminophenol with 25mL water and 2.3mL HCl(aq) to flask A, swirl - add 4.5g sodium acetate trihydrate to 12mL water to flask B, swirl - add 3.3g acetic anhydride to flask A, add flask B to flask A, swirl - let sit 10-15 min, put on ice bath, suction filter crystals, air dry - characterize by IR. Part 2: Phenacetin from Acetaminophen Place acetaminophen1 (1.5g, 0.01mmol, 1 equiv.) and methanol (10mL) in a roundbottom flask (50mL). To this mixture add 50% aq. NaOH (0.6mL, 0.011mmol, 1.1 equiv.) and swirl the mixture to dissolve the crystals. Fit the flask with a reflux condenser then add ethyl bromide (2.2g, 0.02mmol, 1.5mL, 2 equiv.) down the condenser. Heat the mixture gently at reflux for about two hours. At the end of that period, add about 20mL of hot water down the condenser slowly; crystals should begin to appear as the last of the water is added. Remove the condenser and set the flask in ice-water to induce crystallization. Collect the crystals by suction filtration, washing with small portions of cold water. When the crystals are dry, determine yield (1.03g crunchy bright white crystals, 5.75mmol, 57.9%): M.P. 127-128C (lit. 135C). IR assignments 1659cm-1 837cm-1 1611,1557,1509,1447cm-1 1369cm-1 3278cm-1 Amide C=O stretching vib. Aromatic C-H bending vib. (2 adj H atoms) Aromatic C=C stretching vib. Alkane C-H bending vibrations Amide N-H stretching vib. 1 H NMR assignments 1.374 (t, 3H, 3H-1), 1.634 (s, 2H), 2.124 (s, 3H, 3H-6), 3.982 (q, 2H, 2H-2), 6.819 (m, 2H, 2H-4), 7.174 (m, 1H, 1H-5), 7.336 (m, 2H, 2H-3) 13 C NMR assignments 15.128 (1C, R-CH3, 1), 24.554 (1C, R-CH3, 8), 63.997 (1C, R1-CH2-O-R, 7), 115.036 (2C, aromatic 1H, 4 & 5), 131.199 (1C, aromatic 0H, 3), 156.099 (1C, aromatic 0H, 6), 168.753 (1C, R-NH-(C=O)-R1, 2). Outline of Experiment - 1.5g acetaminophen, 10mL MeOH, 0.6ml 50% NaOH.in a 50mL RBF, swirl - Setup for reflux, add 2.2g EtBr down condenser, lightly reflux 2hrs - Add 20mL hot water down condenser - Remove condenser, move to ice bath, suction filter, dry - Characterize by 1H and 13C NMR, IR, m.p. References Merck & Co Inc, “Merck Index”, 12th ed., Whitehouse Station, NJ: 2000 CambridgeSoft Corp, “Chemfinder”: Jan. 7, 2002; http://chemfinder.cambridgesoft.com Wilbert & De Angelis, U.S. pat 2,998,450 (1961 to Warner-Lambert) McMurray J., “Organic Chemistry”, 5th ed; Pacific Grove, CA: 1999 Commentary It is in this writers opinion that to call acetaminophen or phenacetin a non-steroidal antiinflammatory drug (NSAID) because it is a not an opioid analgesic is as incorrect as calling Welbutrin a selective serotonin reuptake inhibitor (SSRI) because it is not a tricylic or monoamine oxidase inhibitor (MAOI) antidepressant!