Dibenzalacetone Synthesis: Lab Procedure & Analysis
advertisement

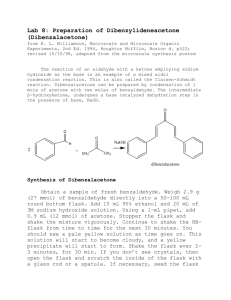
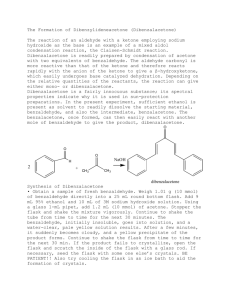
Lab 8: Preparation of Dibenzylideneacetone (Dibenzalacetone) from K. L. Williamson, Macroscale and Microscale Organic Experiments, 2nd Ed. 1994, Houghton Mifflin, Boston d. p522; revised 10/10/98, adapted from the microscale synthesis posted The reaction of an aldehyde with a ketone employing sodium hydroxide as the base is an example of a mixed aldol condensation reaction. This is also called the Claisen-Schmidt reaction. Dibenzalacetone can be prepared by condensation of 1 mole of acetone with two moles of benzaldehyde. The intermediate β-hydroxyketone, undergoes a base catalyzed dehydration step in the presence of base, NaOH. Synthesis of Dibenzalacetone Obtain a sample of fresh benzaldehyde. Weigh 2.26 g (20 mmol) of benzaldehyde directly into a 50-100 mL round bottom flask. Add 15 mL 95% ethanol and 20 mL of 3M sodium hydroxide solution. Using a 1-mL pipet, add 0.9 mL (10 mmol) of acetone. Stopper the flask and shake the mixture vigorously. Continue to shake the RB- flask from time to time for the next 30 minutes. You should see a pale yellow solution as time goes on. This solution will start to become cloudy, and a yellow precipitate will start to form. Shake the flask ever 2-3 minutes, for 30 min. If you don’t see crystals, then open the flask and scratch the inside of the flask with a glass rod or a spatula. If necessary, seed the flask with some one else’s crystals. BE PATIENT!! Also try cooling the flask in an ice bath to aid the formation of crystals. Isolation and Purification Filter the solid from the flask using a Buchner funnel. Wash the crystals 3 X with 5ml each of cold water. Squeeze the product between sheets of filter paper to dry it, and then recrystallize the crude product using a minimum amount of 70:30 mixture of ethanol:water in a 50 mL Erlenmeyer flask. Remove the boiling solution from the hot plate, and place it aside to cool to room temperature. If the product separates as an oil, get a seed crystal (from a neighbor), keep heating the solution to dissolve the oil, and then add the seed crystal. If it still continues to oil out, add a little ethanol and heat and cool again. Keep the flask containing your product on ice till you see crystals form. You may need to scratch your flask with a spatula to initiate crystallization. Collect the product on a Buchner funnel. Wash the crystals once with about 3 mL of icecold 70% ethanol. Dry the product under vacuum by attaching the tube to an aspirator for ~20 minutes. Determine the weight of the dibenzalacetone and its melting point, and calculate the percentage yield. In a typical experiment the melting point should range from l10.5 to 112°C. In the last step of the aldol condensation, loss of water from the β-hydroxyketone can form molecules in which the alkene hydrogen atoms are either cis or trans to each other. The name dibenzalacetone does not completely characterize the molecules made in this experiment. There are 3 isomeric dibenzalacetones, one melting at 100-110 °C, λmax 330 nm, ε 34,300; another melting at 60 °C, λmax 295 nm, ε 20,000; and a third, a liquid with λmax 287 nm ε 11,000. Both the melting points and the UV spectral data give some hints regarding the structures of these molecules. The first one is very symmetrical and can pack well into a crystal lattice. The long wavelength of the ultraviolet light absorption maximum and the high value of the molar absorbance ε indicate a long, planar conjugated system. The other two molecules are increasingly less able to pack nicely into a crystal lattice or to have a planar conjugated system. Post Lab Questions 1. How would you change the procedures in this experiment if you wished to synthesize benzalacetone, C6H5CH=CHCOCH3? Benzalacetophenone, C6H5CH=CHCOC6H5? 2. The product formed has a pale yellow color. Why?