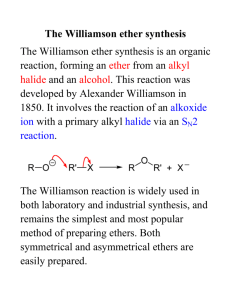
Health Council of the Netherlands
Phenacetin
Evaluation of the carcinogenicity and genotoxicity
Gezondheidsraad
Health Council of the Netherlands
Aan de staatssecretaris van Sociale Zaken en Werkgelegenheid
Onderwerp
Uw kenmerk
Ons kenmerk
Bijlagen
Datum
: aanbieding advies Phenacetin
: DGV/MBO/U-932342
: U-7412/BvdV/fs/246-C17
:1
: 13 november 2012
Geachte staatssecretaris,
Graag bied ik u hierbij het advies aan over de gevolgen van beroepsmatige blootstelling aan
fenacetine.
Dit advies maakt deel uit van een uitgebreide reeks waarin kankerverwekkende stoffen
worden geclassificeerd volgens richtlijnen van de Europese Unie. Het gaat om stoffen
waaraan mensen tijdens de beroepsmatige uitoefening kunnen worden blootgesteld.
Dit advies is opgesteld door een vaste subcommissie van de Commissie Gezondheid en
beroepsmatige blootstelling aan stoffen (GBBS), de Subcommissie Classificatie van
carcinogene stoffen. Het advies is getoetst door de Beraadsgroep Gezondheid en omgeving
van de Gezondheidsraad.
Ik heb het advies vandaag ter kennisname toegezonden aan de staatssecretaris van
Infrastructuur en Milieu en aan de minister van Volksgezondheid, Welzijn en Sport.
Met vriendelijke groet,
prof. dr. W.A. van Gool,
voorzitter
Bezoekadres
Postadres
Parnassusplein 5
Postbus 16052
2 5 11 V X D e n
2500 BB Den
Haag
E - m a i l : b . v. d . v o e t @ g r. n l
Te l e f o o n ( 0 7 0 ) 3 4 0 7 4 4 7
w w w. g r. n l
Haag
Phenacetin
Evaluation of the carcinogenicity and genotoxicity
Subcommittee on the Classification of Carcinogenic Substances of
the Dutch Expert Committee on Occupational Safety,
a Committee of the Health Council of the Netherlands
to:
the State Secretary of Social Affairs and Employment
No. 2012/21, The Hague, November 13, 2012
The Health Council of the Netherlands, established in 1902, is an independent
scientific advisory body. Its remit is “to advise the government and Parliament on
the current level of knowledge with respect to public health issues and health
(services) research...” (Section 22, Health Act).
The Health Council receives most requests for advice from the Ministers of
Health, Welfare & Sport, Infrastructure & the Environment, Social Affairs &
Employment, Economic Affairs, Agriculture & Innovation, and Education,
Culture & Science. The Council can publish advisory reports on its own
initiative. It usually does this in order to ask attention for developments or trends
that are thought to be relevant to government policy.
Most Health Council reports are prepared by multidisciplinary committees of
Dutch or, sometimes, foreign experts, appointed in a personal capacity. The
reports are available to the public.
The Health Council of the Netherlands is a member of the European
Science Advisory Network for Health (EuSANH), a network of science
advisory bodies in Europe.
The Health Council of the Netherlands is a member of the International Network
of Agencies for Health Technology Assessment (INAHTA), an international
collaboration of organisations engaged with health technology assessment.
I NA HTA
This report can be downloaded from www.healthcouncil.nl.
Preferred citation:
Health Council of the Netherlands. Phenacetin. Evaluation of the carcinogenicity
and genotoxicity. The Hague: Health Council of the Netherlands, 2012;
publication no. 2012/21.
all rights reserved
ISBN: 978-90-5549-920-5
Contents
Samenvatting 9
Executive summary 11
1
1.1
1.2
1.3
Scope 13
Background 13
Committee and procedures 13
Data 14
2
2.1
2.2
General information 15
Identity and physicochemical properties 15
IARC classification 16
3
3.1
3.2
Carcinogenicity 17
Observations in humans 17
Carcinogenicity studies in animals 22
4
4.1
Mode of action 27
Genotoxic mode of action 27
Contents
7
5
5.1
5.2
Classification 31
Evaluation of data on carcinogenicity and genotoxicity 31
Recommendation for classification 32
References 33
A
B
C
D
E
F
G
H
I
Annexes 39
Request for advice 41
The Committee 43
The submission letter 45
Comments on the public review draft 47
IARC Monograph 49
Human data 51
Animal data 57
Genotoxicity data 59
Carcinogenic classification of substances by the Committee 61
8
Phenacetin
Samenvatting
Op verzoek van de minister van Sociale Zaken en Werkgelegenheid evalueert en
beoordeelt de Gezondheidsraad de kankerverwekkende eigenschappen van stoffen waaraan mensen tijdens het uitoefenen van hun beroep kunnen worden blootgesteld. De evaluatie en beoordeling worden verricht door de subcommissie
Classificatie van Carcinogene Stoffen van de Commissie Gezondheid en
Beroepsmatige Blootstelling aan Stoffen van de raad, hierna kortweg aangeduid
als de commissie. In het voorliggende rapport neemt de Commissie fenacetine
onder de loep. Fenacetine werd vanaf 1887 tot ongeveer 1980 gebruikt als pijnstiller. Omdat er steeds meer aanwijzingen kwamen dat chronisch gebruik van
fenacetine vormen van nierproblemen kan veroorzaken, is de stof niet meer als
geneesmiddel geregistreerd. Fenacetine wordt vaak versneden aangetroffen in
illegaal verkrijgbare cocaïne.
Op basis van de beschikbare gegevens leidt de commissie af dat fenacetine
kankerverwekkend is voor de mens. Zij beveelt aan om de stof te classificeren in
categorie 1A.* De commissie concludeert verder dat de stof een stochastisch
genotoxisch werkingsmechanisme heeft.
*
Volgens het classificatiesysteem van de Gezondheidsraad (zie bijlage I).
Samenvatting
9
10
Phenacetin
Executive summary
At request of the Minister of Social Affairs and Employment, the Health Council
of the Netherlands evaluates and judges the carcinogenic properties of
substances to which workers are occupationally exposed. The Evaluation is
performed by the subcommittee on the Classification of Carcinogenic
Substances of the Dutch Expert Committee on Occupational Standards of the
Health Council, hereafter called the Committee. In this report, the Committee
evaluated phenacetin. Phenacetin was after the introduction in 1887 up to the
early 1980s used as an analgesic drug. Because chronic use of phenacetin is
suspected to cause renal problems the registration of the drug has been
discontinued. Phenacetin is being used as a cutting agent to adulterate illegally
supplied cocaïne.
Based on the available information, the Committee is of the opinion that
phenacetin is carcinogenic to humans and recommends to classify the substance
in category 1A.* The Committee is furthermore of the opinion that phenacetin
acts by a stochastic genotoxic mechanism.
*
According to the classification system of the Health Council (see Annex I).
Executive summary
11
12
Phenacetin
Chapter
1.1
1
Scope
Background
In the Netherlands a special policy is in force with respect to occupational use
and exposure to carcinogenic substances. Regarding this policy, the Minister of
Social Affairs and Employment has asked the Health Council of the Netherlands
to evaluate the carcinogenic properties of substances, and to propose a
classification (see Annex A). In addition to classifying substances, the Health
Council also assesses the genotoxic properties of the substance in question. The
assessment and the proposal for a classification are expressed in the form of
standard sentences (see Annex I).
This report contains the evaluation of the carcinogenicity of phenacetin
1.2
Committee and procedures
The evaluation is performed by the subcommittee on the Classification of
Carcinogenic Substances of the Dutch Expert Committee on Occupational
Standards of the Health Council, hereafter called the Committee. The members
of the Committee are listed in Annex B. The submission letter (in English) to the
State Secretary can be found in Annex C.
In June 2012, the President of the Health Council released a draft of the
report for public review. No comments were received on the draft document.
Scope
13
1.3
Data
The evaluation and recommendation of the Committee is based on scientific
data, which are publicly available. The starting points of the Committees’ reports
are, if possible, the monographs of the International Agency for Research on
Cancer (IARC). This means that the original sources of the studies, which are
mentioned in the IARC-monograph, are reviewed only by the Committee when
these are considered most relevant in assessing the carcinogenicity and
genotoxicity of the substance in question. The evaluation of the carcinogenicity
of phenacetin has been based on IARC evaluations (IARC volume 13 (1977),
IARC volume 24 (1980), IARC supplement 7 (1987) and IARC volume 100A
(2011))1-4 (in Annex E a summary is given of the IARC data) and additional
scientific data, which are publicly available. Additional data were obtained from
the online databases Toxline, Medline and Chemical Abstracts covering the
period 1978 to September 2012 using phenacetin and CAS no 62-44-2 as key
words in combination with key words representative for carcinogenesis and
mutagenesis. The new relevant data were included in this report.
14
Phenacetin
Chapter
2.1
2
General information
Identity and physicochemical properties
Chemical name
CAS registry number
EINECS-number
EEC-number
RTECS-number
Synonyms
Appearance
:
:
:
:
:
:
:
Occurrence
Use
:
:
Molecular formula
Structural formula
:
:
Molecular weight
Boiling point
Melting point
Vapour pressure
Vapour density (air = 1)
Solubility
Stability and reactivity
EU Classification
:
:
:
:
:
:
:
:
General information
N-(4-ethoxyphenyl)acetamide1
62-44-2
200-533-05
N-(4-ethoxyphenyl), acetyl-phenetidine, 1-acetamido-4-ethoxybenzene
odorless, white, glistening crystals, usally scales or as fine white,
crystalline powder6
analgesic and antipyretic drug in human and veterinary medicine.2;
registration in the Netherlands was discontinued in 1984 because of
serious side effects on the kidney;
illegal use as adulterant in cocaine powder
C10-H13-N-O26
179.226
242-245°C6
134-135°C6
Slightly soluble in water (1 in 1,300)2
Unstable to oxidizing agents, iodine and nitrating agents2
Not classified in Annex I of Directive 67/548/EEC
15
2.2
IARC classification
In 2011, IARC concluded :
There is sufficient evidence in humans for the carcinogenicity of analgesic
mixtures containing phenacetin. Analgesic mixtures containing phenacetin cause
cancer of the renal pelvis, and of the ureter.
There is limited evidence in experimental animals for the carcinogenicity of
analgesic mixtures containing phenacetin.
There is sufficient evidence in humans for the carcinogenicity of phenacetin.
Phenacetin causes cancer of the renal pelvis, and of the ureter.
There is sufficient evidence in experimental animals for the carcinogenicity
of phenacetin.
Analgesic mixtures containing phenacetin are carcinogenic to humans
(Group 1). Phenacetin is carcinogenic to humans (Group 1).
16
Phenacetin
Chapter
3.1
3
Carcinogenicity
Observations in humans
Many case report studies showed the existence of renal pelvic and other
urothelial tumours in patients who have used large amounts of phenacetincontaining analgesics.7-16, 17-22
A vast amount of case-control studies23-28, 29-44 have been published. These
studies show that phenacetin-containing analgesics are part of the etiology of
renal pelvic, urothelial and bladder cancer. Most of the exposed individuals in
these case-control studies are exposed to phenacetin-containing analgesics,
which makes it difficult to investigate the effect of exposure to phenacetin only.
Most of the studies were published 15-20 years ago, due to the fact that
phenacetin-containing products had been off the market in most countries for
decades now. Recent studies were not published because the lack of long-time
phenacetin users. The case-control studies have been summarized in the
following paragraphs and in Annex F.
Renal pelvis cancer
McCredie et al. (1986) conducted a hospital based case-control study in New
South Wales, Australia to investigate the risk factors for renal cancer. Sixty six
cases of renal pelvis cancer, 86 cases of renal parenchyma cancer and 751
controls were collected between 1970 and 1982 in Sidney, Australia. Information
Carcinogenicity
17
on consumption of phenacetin-containing analgesics was obtained through
completion of a structured questionnaire at interview. Pathologists classified the
tumours according to their histological appearances and sought evidence of
‘intermediate’ or ‘advanced’ renal papillary necrosis (RPN). Cases were
excluded if the presence or absence of RPN could not be established. RPN and
regular consumption of phenacetin both increased the risk for renal pelvis cancer.
The risk of renal pelvis cancer increased nearly 4 times for regular consumers of
phenacetin without RPN (RR: 3.6, 95% CI: 1.6-8.1) and 20 times for regular
consumers of phenacetin with RPN (RR: 20, 95% CI: 12-34), compared to nonconsumers without RPN.36
McCredie et al. (1988) also conducted a population-based case control study
in New South Wales, Australia to investigate the risk of developing renal cancer
papillary necrosis and cancer of the renal pelvis, ureter or bladder associated with
consumption of either phenacetin or paracetamol. Data were acquired from 381
cases (identified between 1978 and 1982) and 808 controls. The risk of cancer of
the renal pelvis was statistically significantly increased nearly 6 and 8-fold with a
lifetime consumption of respectively, > 0.1 kg (OR: 5.7, 95% CI: 3.2-10.0) and
> 1 kg (OR: 7.9, 95% CI: 4.6-13.8) phenacetin.37
In another population-based case control study in New South Wales,
Australia, McCredie et al. (1993) investigated the consumption of phenacetin
and paracetamol and the risk of cancer of the kidney and renal pelvis, using data
of 489 cases of renal-cell cancer and 147 cases of renal pelvic cancer diagnosed
in 1989 and 1990, together with 523 controls from the electoral rolls. A doserelated increase in the risk of cancer of the renal pelvis was observed in
consumers of phenacetin/aspirin compounds. When used according to the
definition of “taken at least 20 times in lifetime” phenacetin/aspirin compounds
increased the risk of renal pelvic cancer more than a 12-fold (RR: 12.2, 95% CI:
6.8-22.2).39
McLaughlin et al. (1985) conducted a population-based case-control study of
renal cancer (495 cases of renal cell cancer, 74 cases of renal pelvis cancer and
697 controls) in Minneapolis, USA. Patients were collected in the period 19741979. Patients and the control group were interviewed in 1980 about the use of
analgesic drugs. Information of different variables was obtained, including the
use of analgesic drugs (phenacetin-containing, acetaminophen-containing and
aspirin). A drug was considered phenacetin-containing if phenacetin was
included in the formulation from 1955 to 1974. Exposures after 1973 were
excluded for analysis. The groups were divided in male/female and in never,
ever, irregular and regular (subdivided in ≤ 36 months and > 36 months) users.
Long-term regular use of phenacetin-containing drugs was associated with an
18
Phenacetin
increase in risk for renal pelvic cancer among males (OR: 8.1, 95% CI: 1.2-62),
but not among females (4.2, 95% CI: 0.4-42).41
Pommer et al. (1999) conducted a case-control study in the area of the former
West Berlin, including 647 new diagnosed cases of urethelial cancer (571
bladder, 25 ureter and 51 renal pelvis cancer cases) from eight hospitals of the
study area between 1990 and 1995 and 647 population-based controls. Intake of
more than 1 kg phenacetin in analgesic mixtures was associated with an
increased risk (not significantly) of renal pelvic cancer (OR of 5.3, 95% CI:
0.3-81).43
Ureter cancer and/or renal pelvis cancer
Several of the case-control studies (including two studies which are already
described above by McCredie et al.,1988, Pommer et al., 199937,43) also analysed
the risk of phenacetin-containing analgesics consumption for the development of
ureter cancer (alone or together with renal pelvic cancer). In the populationbased case-control study in New South Wales, Australia by McCredie et al.
(1988)37 no association was found between ureter cancer and a lifetime
consumption of > 0.1 kg (OR: 0.7, 95% CI: 0.3-2.2) or > 1 kg phenacetin (OR:
1.2, 95% CI: 0.5-3.0).
In the case-control study in the area of the former West Berlin by Pommer et
al. (1999)43 no association was found between the number of renal pelvis cancer
and ureter cancer combined and a lifetime intake of more than 1 kg phenacetin in
analgesic mixtures (OR of 1.8, 95% CI: 0.2-13).
Jensen et al. (1989)33 conducted a case-control study (96 cases and 294
controls, identified between 1979 and 1982) in Denmark to investigate the risk of
analgesic intake (phenacetin and/or aspirin) and cancer of the renal pelvis and
ureter. Seventy nine percent of the tumours were located in the renal pelvis
(including calyces). There was an indication of a dose-effect relationship for
phenacetin-containing analgesics and cancer of the renal pelvis and ureter. A
statistically significant increase in relative risk (RR) was seen for female users of
phenacetin-containing analgesics (RR: 4.2, 95% CI: 1.5-12.3), but not for male
users (RR: 2.4, 95% CI: 0.9-6.8).33
Linet et al. (1995) investigated 502 cases (308 renal pelvis cancer and 194
ureter cancer, identified between 1983 and 1986) and 496 controls in a
population-based case-control study in New Jersey, Iowa and Los Angeles, USA.
Neither cumulative lifetime ingestion nor duration of regular use of phenacetin,
whether alone or in combination with acetaminophen or aspirin, was associated
with significantly increased risk of renal pelvis and ureter cancer. Although this
Carcinogenicity
19
study contained a large amount of cases, it only contained small number of
regular analgesic users.35
Renal cell cancer
Three case-control studies on renal pelvis cancer, which are already described
above, also analysed the risk of phenacetin-containing analgesics consumption
for the development of renal cell cancer.36,41.
In the population-based case-control study in Minneapolis, US of
McLaughlin et al. (1985)41 (described above), long-term regular use of
phenacetin-containing drugs was associated with a statistically significant
increase in risk for renal cell cancer in women (OR: 1.7, 95% CI: 1.1-2.7 for
ever-users and OR: 1.7, 95% CI: 1.1-2.6 for irregular-users compared to never
users).
In another population-based case-control study by McLaughlin et al.(1992)42
in Shanghai, China (154 cases and 157 controls) regular use of phenacetincontaining analgesics (at least 2 times a week for a period of at least 2 weeks)
was not associated with renal cell cancer (OR: 2.3, 95% CI: 0.7-7.0).
In the hospital based case-control study in New South Wales, Australia of
McCredie et al. (1986)36 (described above), regular use of phenacetin-containing
analgesics increased the risk of cancer of the renal parenchyma (RR: 2.5, 95%
CI: 1.3-4.9.), but was not increased by the presence of renal papillary necrosis
(RPN). Thus, unlike renal pelvis cancer, the relationship between consumption
of phenacetin-containing analgesics and renal parenchyma appears to be a direct
one without any intervening effect of RPN.
In the population-based case-control study in New South Wales, Australia by
McCredie et al. (1993) (described above), no association was found between the
number of renal-cell cancers and consumption of phenacetin/aspirin compounds
(RR: 1.4, 95% CI: 0.9-2.3).39
In another study McCredie et al. (1995)40 pooled data from 1,313 cases and
1724 controls from Australia, Denmark, Germany, Sweden and the US,
identified between 1989 and 1991. The role of phenacetin-containing and other
types of analgesics in the development of renal-cell cancer was studied. Relative
risks, adjusted for the effects of age, sex, body-mass index, tobacco smoking and
study centre, were not statistically significantly increased with a lifetime
consumption of > 0.1 kg phenacetin (or when subjects were subdivided further
by amount). According to the authors, these findings do not support the
hypothesis that analgesics containing phenacetin increase the risk, although the
20
Phenacetin
number of ‘regular’ users and the amount of analgesics consumed were too small
to confidently rule out a minor carcinogenic effect of phenacetin.
Kreiger et al. (1993) performed a population-based case-control study in
Ontario, Canada of risk factors for renal cell carcinoma. Data were collected on
518 case and 1,381 controls identified between 1986 and 1987. In this large
study different risk factors for renal cell carcinoma were observed. No
association was found between phenacetin-only use (5 cases, 9 controls) and the
risk of renal cell carcinoma (OR: 2.5, 95% CI: 0.3-18.5 for males and OR: 1.8,
95% CI: 0.5-7.3 for females) or between acetaminophen-only use and the risk of
renal cell carcinoma (OR: 0.8, 95% CI: 0.3-1.7 for males and OR: 0.9, 95% CI:
0.5-2.0 for females), although few subjects used either compound.34
Gago et al. (1999) conducted a population-based case-control study in Los
Angeles, US (1,204 cases and equal number controls) to investigate the
relationship between sustained use of analgesics and the risk of renal cell
carcinoma. Regular use of analgesics (2 or more times a week for 1 months or
longer) was a significant risk factor for renal cell carcinoma for all four major
classes of analgesics (aspirin, non-steroidal anti-inflammatory agents other than
aspirin, acetaminophen and phenacetin). Regular use of phenacetin containing
analgesics was associated with an OR of 1.9 (95% CI: 1.3-2.7). A dose-related
increase in risk of renal cell carcinoma was observed after further subdivision
into different amounts of the maximum weekly dose.32
Bladder cancer
Several epidemiological studies 23,25,27,29-31,43 have examined phenacetin and
bladder cancer. Two of the case-control studies on renal pelvis and ureter cancer
which are already described above, also analysed the risk of phenacetincontaining analgesics consumption for the development of bladder cancer
(McCredie et al., 1988; Pommer et al., 1999).37,43
In the population-based case-control study in New South Wales, Australia by
McCredie et al. (1988)37(described above), risk for cancer of the bladder was
doubled by the consumption of phenacetin (OR: 2.0, 95% CI: 1.1-3.5 for subjects
with a lifetime consumption of > 1 kg phenacetin and OR: 2.1, 95% CI: 1.3-3.5
for subjects with a lifetime consumption of > 0.1 kg phenacetin).
In the case-control study in Berlin, Germany by Pommer et al. (1999)43
(described above), no association was observed between a lifetime intake of
more than 1 kg phenacetin in analgesic mixtures and bladder cancer (OR: 0.75,
95% CI: 0.39-1.43).
Carcinogenicity
21
In a population-based case-control study conducted in Los Angeles,
California, US by Castelao et al. (2000), 1,514 cases of bladder cancer and an
equal number of controls, identified between 1987-1996 were investigated.
Regular use of analgesics was not associated with an increased risk of bladder
cancer in either man or women. The intake of phenacetin-containing analgesics
was positively related to bladder cancer risk in a dose-dependent manner, while
the intake of its major metabolite in humans, acetaminophen, was unrelated to
risk. Regular use of phenacetin-containing analgesics was not associated with an
increased risk of bladder cancer (OR: 1.5, 95% CI: 0.85-2.73).29
In a hospital based case-control study conducted in Spain by Fortuny et al.
(2006), the use of non-aspirin non-steroidal anti-inflammatory drugs (NSAID),
aspirin, paracetamol (acetaminophen), phenacetin, and metamizol (dipyrone) and
risk of bladder cancers was assessed. Data on 958 cases and 1,029 controls,
identified between 1997 and 2000 was analysed. A significant reduction in
bladder cancer risk was observed for regular users of non-aspirin NSAIDs
compared with never users. No evidence of an overall effect for regular use
paracetamol or aspirin was observed. Regular use of phenacetin was not
associated with an increased risk of bladder cancer (OR: 1.3, 95% CI: 0.3-4.5).
However, this estimate was based on only 7 cases and 12 controls.30
In a population-based case-control study conducted in New Hampshire, UK
by Fortuny et al. (2007), the influence of phenacetin, other analgesics and
NSAID use on the risk of bladder cancer was investigated. Data from 376 cases
and 463 controls, identified between 1998 and 2001 was analysed. Elevated
OR’s were associated with reported use of phenacetin-containing medications
(OR: 2.2, 95% CI: 1.3-3.8 for ever compared to never users), especially with
longer duration of use (OR: 3.0, 95% CI: 1.4-6.5 for > 8 years of use).31
3.2
Carcinogenicity studies in animals
A group of 30 BD I and BD III rats (age, 100 d) received phenacetin (40-50 mg)
daily in the diet (average total, dose 22g). One rat died after a total dose of 10 g
and was found to have an osteochondroma. The mean age of death of the treated
animals was 770 days, the control animals 750 days. No tumours related to
treatment were observed.45
Four groups of 15, 20, 20, and 24 male albino rats were fed with diets
containing 0, 0.05, 0.1 or 0.5 % N-hydroxyphenacetin (metabolite of phenacetin)
during 73 weeks. Assuming a body weight of 400 grams and a daily food intake
of 20 grams, the exposure of N-hydroxyphenacetin was 25, 50, and 250 mg/kg
bw/day respectively. Of treated animals 11, 13 and 15 rats were still alive at the
22
Phenacetin
time of appearance of the first tumour after 45, 45 and 38 weeks. Of these
animals 8/11, 13/13 and 15/15 developed liver tumours (described as
hepatocellular carcinomas). None of the control group animals developed
tumours. One of the animals fed with 0.1% diet developed a transitional cell
carcinoma of the renal pelvis.46
Female SD rats were given 0 or 0.535% phenacetin in the diet for 86 or 110
weeks. Assuming a body weight of 400 grams and a daily food intake of 20
grams the exposure of phenacetin was 268 mg/kg bw/day. In the 86-week study,
epithelial hyperplasia of renal papillae was found in 2/24 controls and 21/38
treated animals. In the 110 week study the following changes were observed:
Urothelial hyperplasia of the renal papillae in 26 animals, dilatation of vasa recta
in 28, and epithelial hyperplasia in 1 animal. In addition, carcinomas of the
mammary gland (5/30) and ear duct (4/30; P>0.05) were found in the treated
group. In the control group, uroepithelial hyperplasia was found in 5 animals,
dilatation of vasa recta in 8 and mammary carcinoma in 1 animal.47
Two groups of SD rats (50 male, 50 female, age 9 wks) were fed a diet
containing 1.25 or 2.5% phenacetin for 18 months, followed by a basal diet for 6
months. Assuming a body weight of 400 grams and a daily food intake of 20
grams the exposure of phenacetin was 625 and 1,250 mg/kg bw/day respectively.
The control group (65 male and 65 female) were fed with the same basal diet.
Among animals surviving for 24 months or dying within 24 months with
tumour(s), neoplasms were detected in 27/27 males and 21/27 females fed 2.5%,
in 20/22 males and 19/25 females fed 1.25% and in 1/19 males and 6/25 females
in the control group. Tumours (benign and malignant) of the nasal cavity were
found in 16/27 males and 7/27 females fed 2.5% and in 16/22 males and 6/25
females fed 1.25%. Malignant tumours of the urinary tract were detected in
13/27 males and 4/27 females fed with the high dose and in 1/22 males and 0/25
females fed with the low dose; 2 papillomas were found in females given the
high dose. No nasal cavity or urinary tract tumours were seen in controls.48
Two groups of B6C3F1 mice (52 male and female, age 6 weeks) were fed for
96 weeks a diet containing 1.25 or 0.6% phenacetin followed by a basal diet for 8
weeks. Assuming a body weight of 20 grams and a daily food intake of 3 grams
the exposure of phenacetin was 1,875 and 900 mg/kg bw/day respectively. The
control group of animals (50 mice of each sex) was fed the same basal diet for
104 weeks. All animals were killed at the end of the experiment. The organs
were examined histopathologically. Mice that died during the experiment were
also autopsied.
Phenacetin at a dose of 0.6% induced a significant increased incidence of
renal cell adenoma in male mice only. A dose of 1.25% was induced a significant
Carcinogenicity
23
increase in both renal cell adenoma and carcinoma in male mice. A clear doseresponse relationship was seen between the doses of phenacetin and the
induction of renal cell carcinoma. A statistically significant increased incidence
of tumours was found in the liver, lung, skin, hematopoietic system (leukaemia
or lymphoma) and occasionally in some other organs.49
Four groups of twenty rats (male Sprague-Dawley, age 6 weeks) were given
phenacetin (0, 0.5, 1.0 or 1.5 %) in the diet for 6 or 12 weeks. The 0.5, 1.0 and
1.5 % groups had a real phenacetin intake of 0.78, 1.28 and 1.77 g/kg bw (at
week 2 of the experiment) and this intake decreased to 0.31, 0.65 and 1.18 g/kg
bw (at week 12).Ten rats of each group were killed at 6 and 12 weeks. One hour
before killing a single i.p injection of labelled thymidine was given. To
determine to which extent the labelled thymidine was incorporated in the DNA
of various tissues, the labelling index was measured. A high labelling index
indicates a high cell proliferation. There was a dose-related increase in the
labelling index in the urothelium of the bladder and kidney (especially after 6
weeks and 1.0% and 1.5% dose). After 6 weeks the labelling indices were
increased in the bladder. After 12 weeks the labelling indices in the bladder were
only increased numerically but not statistically significant. In the renal pelvic the
labelling index was significantly increased at doses of 1.0 and 1.5 %. At week 12
the majority of rats treated with 1.5% had labelling indices ≥ 2-fold than the
control both in kidney and bladder. The increased labelling indices were
associated with urothelial hyperplasia (in particular after 6 weeks).50
Twenty male Crl:CDBR rats were treated by gavage with phenacetin during
7 or 14 days. The rats were divided in 4 groups: a control, a low-dose (100 mg/kg
bw/day), an intermediate (625 mg/kg bw/day) and a high-dose group (1,250
mg/kg bw/day). One week of phenacetin treatment resulted in dose-related
increases in DNA synthesis in both respiratory and olfactory mucosa. The
increase observed in the respiratory mucosa was due to inflammatory cells in the
lamina propria and not to proliferation of the respiratory epithelial cells. One or
two weeks of daily phenacetin treatment resulted in degenerative changes in the
olfactory epithelium and necrosis of Bowman’s glands. These changes were
associated with increases in cell proliferation in the olfactory epithelium only.
Two-week daily gavage treatment of rats with phenacetin at 100, 625 and 1,250
mg/kg/day increased olfactory epithelial cell replication by 62.1, 174 and 763%,
respectively.51
Phenacetin was mixed in the feed at a concentration of 0.7 or 1.4% and
administered to transgenic CB6F1-rasH2 mice and non-transgenic, wildtype
(non-Tg, WT) mice during 24 weeks. Assuming a body weight of 20 grams and a
daily food intake of 3 grams the exposure to phenacetin was 1,050 and 2,100
24
Phenacetin
mg/kg bw/day respectively. Phenacetin induced spleen haemangiosarcoma and
lung adenomas in the rasH2mice but not in the non-Tg mice. Lung adenomas
(12 in exposed versus 2 in control) and spleen hemangiosarcomas (6/0) were
found in male rasH2 treated with 1.4% phenacetin in the feed. This incidence
was significant higher than in the corresponding non-Tg mice.52
P53+/- transgenic mice were given phenacetin by daily gavage with dose of
100, 200 and 350 mg/kg bw/day suspended in 0.5% methylcellulose during 26
weeks. In a separate study the mice were given a dose of 0.14, 0.7 and 1.4%
phenacetin in the diet. Control and high-dose groups of wild-type mice were
included in both studies. No increase in treatment-related tumour incidence was
found after 26 week of treatment.53
The transgenic Tg.AC mice strain is able to respond to dermal application
with development of squamous-cell papillomas of the skin. Phenacetin was
administered topically (0, 0.08, 0.4 and 2 mg, daily) and in the diet (0, 12, 60,
300 ppm) during 26 weeks. Phenacetin was negative by both routes of
exposure.54
Phenacetin was administered in the feed (0, 0.1, 0.25, 0.5, or 0.75% w/w) to
transgenic Xpa-/- mice (15 male, 15 female), to double transgenic Xpa-/-/p53+/mice (15 male, 15 female) and to wild type (WT) C57BL/6 mice (15 male, 15
female). Assuming a body weight of 20 grams and a daily food intake of 3 grams
the exposure of phenacetin was 150, 375, 750, 1,125 mg/kg bw/day respectively.
The exposure to phenacetin was 39 weeks for all groups. At the end of the
experiment renal proximal tubular hyperplasia was observed in two high-dose
Xpa-/- males and in one Xpa-/-/p53+/- male mouse. A tubular adenoma was found
in a Xpa-/-/p53+/- female mouse. In all male and female transgenic, but not the
WT mice, multifocal karyomegaly in the proximal renal tubules was found. In
addition, olfactory epithelial degeneration was observed in the nose of most male
and female transgenic and WT mice of the high-dose groups.55
Phenacetin had the ability to induce morphological transformation in
cultured
C3H/10T1/2 clone 8 mouse embryo cells (10T1/2 cells). Treatment of the
10T1/2 cells with 0.5, 1.0, and 2.0 mg/ml phenacetin caused a dose-dependent
decrease in plating efficiency and a dose-dependent increase in type II
morphologically transformed foci.56
Phenacetin tested in the Syrian hamster embryo transformation assay gave
negative results. The highest concentration phenacetin tested was 500 µg/ml
phenacetin. Phenacetin above a concentration level of 500 µg/ml was insoluble
in the medium with DMSO.57
Carcinogenicity
25
In an initiation-promotion experiment male F344 rats (6 weeks of age) were
divided in two groups of 20 and one of 10 rats. The two groups of 20 rats were
pretreated with 0.1% DHPN in drinking water and 3.0% uracil in the diet during
4 weeks. DHPN (dihydroxy-di-N-propylnitrosamine) is a carcinogen which is
known to induce tumours of the renal pelvis, renal tubular cells and urinary
bladder in rats. One week after cessation, one group received basal diet and one
group received a diet containing 2.0% phenacetin (average intake 1,145 mg/kg/
day) during the following 35 weeks. The group of 10 animals was given, during
the same period, a diet with 2.0% phenacetin (average intake 1,068 mg/kg/day)
without the initial combination treatment of DHPN and uracil. The occurrence of
renal cell tumours was increased in the group given phenacetin (9/20) as
compared with the DHPN + uracil alone control (1/19). In the urinary bladder,
phenacetin treatment was associated with increased incidence of preneoplastic or
neoplastic lesions. The group of animals, treated with phenacetin alone, without
the pretreatment, induced simple hyperplasias of the urinary bladder at high
incidence.58
26
Phenacetin
Chapter
4.1
4
Mode of action
Genotoxic mode of action
More details of these studies have been summarized in Annex H.
4.1.1
Gene mutation assays
In vitro
Phenacetin was not mutagenic in several bacterial models in the presence or
absence of rat or mouse liver microsome preparations: the models included a
repair test in Bacillus subtillus59 and reverse mutation test in Salmonella
typhimurium TA1535, TA 1537, TA98 and TA 10060,61, Escherichia coli
K 12/343/1361, and B. subtilis TKJ 5211.59 Positive bacterial mutagenic results
have been obtained in S. typhimurium TA 100 in the presence of hamster, but not
rat, liver post-mitochondrial supernatant of Aroclor-treated animals.62-64
Phenacetin led to an increase in the mutant frequency in Salmonella typhimurium
TA 100 in the presence of a hamster liver metabolic activation.65,66
In the hprt test phenacetin induced an increase in the mutant frequency in
V79 Chinese hamster cells in vitro in the presence of hamster liver microsome
preparations.65,67.
Mode of action
27
In vivo
Phenacetin was negative in an intrasanguineous host-mediated assay with E.coli
K 12 in NMRI mice given 2 mmol/kg intraperitoneally. Phenacetin did not
induce an increased frequency of sex-linked recessive lethals in Drosophila
melanogaster.
Phenacetin was given in the feed of DNA repair deficient (Xpa-/- and Xpa-/-/
Trp53+/-) mice and wild type (WT) carrying the IacZ (0.75% w/w, during 0, 4, 8,
or 12 weeks). Xpa-/- mice lack the normal nucleotide excision repair pathway.
Due to this deficiency, these mice are more sensitive to genotoxic compounds
than wild type mice. Phenacetin exposure induced an increase in the lacZ mutant
frequency in the kidney of WT, Xpa-/- and Xpa-/-/Trp53+/- mice as compared with
concurrent untreated controls of the wild type C57BL/6 mice. The increase in
Xpa-/- and Xpa-/-/Trp53+/- mice was stronger than in WT mice. A minor and
negative response was found in the liver and the spleen, respectively. The
observed phenacetin-induced mutant frequency was higher in male than in
female mice.68
4.1.2
Cytogenetic assays
In vitro
Phenacetin induced DNA fragmentations in an acellulair test-system with λ
DNA but not with calf thymus DNA.69
In vivo
No data were available on the genetic and related effects of phenacetin in
humans.
The results of studies on the induction of chromosomal aberrations, sister
chromatid exchanges and micronuclei in rodents treated with phenacetin in vivo
were equivocal.61,70 Phenacetin exposure did not result in an enhanced number of
micronucleated erythrocytes in the bone marrow of NMRI mice given 2 x 5
mmol/kg bw intraperitoneally.61
Following in vivo treatment, the alkaline elution assay showed no increase of
DNA damage in bone-marrow cells of i.p-treated mice or in liver cells of rats
treated by gavage. However, an increase of DNA damage was observed in liver
of rats after i.p. administration of phenacetin and in kidney of rats receiving
28
Phenacetin
phenacetin by gavage.65 Sister chromatid exchanges were seen in mice (i.p, 330
mg/kg bw) treated with phenacetin. This increase of SCE was weak but
statistically significant.65
The micronucleus bone marrow test showed a positive response in mice given
phenacetin i.p. Phenacetin doses of 37.5, 75, 150, 300, 400 and 600 mg/kg bw/day
were administered only once or multiple times (2-4) to CD-1 mice. Positive
responses were seen at 600 mg/kg/day after single and triple dosing and at 400
and 600 mg/kg/day after double dosing.71,72 A single dose of phenacetin of 0, 2, 5,
50 and 100 mg/kg given i.p to SJL Swiss mice resulted in a moderate but
significant increase of cells with micronuclei compared with the control group.73
The micronucleus assay with peripheral reticulocytes from phenacetin-treated
mice (CD-1 and MS/Ae strain) was negative after a single dose of 400, 600 and
800 mg/kg bw(24 h after i.p). Positive results were obtained with 600 and 800
mg/kg bw after 48 h. Double treatment (24 h between treatments) enhanced the
responses. A dose response was obtained for all different sample times. In this
same experiment CD-1 mice treated with phenacetin (i.p, 600 mg/kg bw, single
and double treatment) gave a positive result in the micronucleus test in bone
marrow cells.74
Phenacetin was administered to rats (Sprague-Dawley) with doses of 500,
1,000 and 2,000 mg/kg bw/day during 2 days or 250, 500, 750, 1,000 mg/kg
bw/day during 14 days. Blood samples were taken on day 1, 3, 6, 9, 12 and 15 for
the micronucleus assay with peripheral reticulocytes. In the 14-day test,
phenacetin increased the frequency of micronucleated reticulocytes in peripheral
blood at 500 mg/kg bw/day starting from day 9, and at 750 and 1,500 mg/kg
bw/day starting from day 6. In the test with 2 days application the frequencies of
micronucleated reticulocytes increased at 1,000 and 2,000 mg/kg bw/day. In the
test with 14 days application the micronucleus assay in the bone marrow showed
a positive dose-related response.75
4.1.3
Miscellaneous
In vitro
Hepatocytes isolated from mouse, hamster, rat and guinea pig showed no marked
increase in unscheduled DNA synthesis (UDS) after exposure to phenacetin.76
After treatment with phenacetin, mouse L-cells gave positive results using a
DNA-synthesis inhibition test system.77 An increase in DNA damage measured
by the alkaline elution assay was not observed when human and rat hepatocytes
were treated with phenacetin in vitro.78
Mode of action
29
30
Phenacetin
Chapter
5.1
5
Classification
Evaluation of data on carcinogenicity and genotoxicity
The Committee is aware that in most of the epidemiological studies described
above the effect of phenacetin may be influenced by other analgetic
comedications, by selection bias, especially in the hospital-based case-control
studies, and recall bias. However, the Committee is also of the opinion that the
epidemiological evidence cannot exclude that phenacetin-containing analgetics
are part of the etiology of renal pelvic, urothelial and bladder cancer. However,
the evidence is considered sufficient by the Committee. For bladder cancer the
evidence does not support such a relationship. Based on the available
information the Committee concludes that there is sufficient evidence for
carcinogenicity of phenacetin to humans.
Phenacetin induced tumours of the urinary tract (in mice and rats) and nasal
cavity (in rat) when given orally. New published data consisted of 9 not standard
carcinogenicity studies, which support this conclusion. Three of these studies
with rats gave insight in the mechanism of the damage induced by phenacetin.
They gave evidence of DNA damage in the bladder or nasal mucosa. Four other
studies used transgenic mice. In two of these studies, the transgenic mice showed
increased lung, spleen and kidney tumours compared to wild type mouse. The
two other studies are transformation tests with mouse-embryo and hamster
embryo cells, of which only the study in mouse-embryo showed increased
transformation. Considering the available animal data, the Committee concludes
Classification
31
that there is sufficient evidence for carcinogenicity of phenacetin to animals. In
addition, the Committee is aware that both animal data and the human data show
a relationship beween phenacetin and cancer of the kidney. This relationship was
even more supported by the observation that phenacetin increased the lacZ
mutant frequency in kidney of transgenic mice. Such an analogy in cancer
development in man and animal on the level of a specific organ supports the role
of phenacetin as a carcinogen.
Phenacetin was negative in almost all in vitro bacterial mutagenicity tests. On
the other hand, DNA damage was observed in mammalian cells in vitro and in
vivo. Phenacetin induced inhibition of DNA synthesis and an increase in the
mutant frequency in a gene mutation assay with mammalian cells when hamster
but not rat S9 mix was used as metabolic activation. The positive findings in
vitro were confirmed in in vivo genotoxicity tests. Phenacetin was positive in
several micronucleus tests as well as in a gene mutation test with transgenic
animals; in several studies a clear dose-response relationship was observed.
Therefore, it can be concluded that phenacetin is a stochastic genotoxic
compound.
5.2
Recommendation for classification
The Committee concludes that phenacetin is carcinogenic to humans and
recommends classifying the substance in category 1A.*
Moreover, the Committee concludes that phenacetin has a stochastic genotoxic
working mechanism.
*
According to the classification system of the Health Council (see Annex I).
32
Phenacetin
References
1
IARC. Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42.
IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 1987; Suppl 7: 1-440.
2
IARC. Some miscellaneous pharmaceutical substances. IARC Monographs on the Evaluation of the
Carcinogenic Risks to Humans: 1977; 13: 1-255.
3
IARC. Some miscellaneous pharmaceutical substances. IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans 1980; 24: 135-161.
4
IARC. A review of human carcinogens: Pharmaceuticals. IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans 2011; 100A: 370-400.
5
ESIS. European Chemical Substances Information System (http://esis.jrc.europa.eu/), accessed
September. 2012.
6
HSDB. Hazardeous Substances Data Bank (http://toxnet.nlm.nih.gov/), accessed September. 2012.
7
Blohme I, Johansson S. Renal pelvic neoplasms and atypical urothelium in patients with end-stage
analgesic nephropathy. Kidney Int 1981; 20(5): 671-5.
8
Christensen TE, Ladefoged J. [Uroepithelial tumors in patients with contracted kidneys and massive
abuse of analgesics (phenacetin)]. Ugeskr Laeger 1979; 141(51): 3522-3524.
9
Gonwa TA, Corbett WT, Schey HM, Buckalew VM, Jr. Analgesic-associated nephropathy and
10
Kliment J. [Renal pelvis tumours and abuse of analgesics (author's transl)]. Bratisl Lek Listy 1979;
11
Marek J, Hradec E. Chronic sclerosing ureteritis and nephrogenic adenoma of the ureter in analgesic
transitional cell carcinoma of the urinary tract. Ann Intern Med 1980; 93(2): 249-252.
72(6): 708-713.
abuse. Pathol Res Pract 1985; 180(5): 569-575.
References
33
12
Mihatsch M J, Manz T, Knusli C, Hofer H O, Rist M, Guetg R et al. Phanacetin abuse III. Malignant
urinary tract tumors in phenacetin abuse in Basle 1963-1977 Original Title: Phenacetinabusus III.
Maligne Harnwegtumoren bei Phenacetinabusus in Basel 1963-1977. Schweiz Med Wochenschr
1980; 110(7): 255-264.
13
Mihatsch MJ, Brunner FP, Korteweg E, Rist M, Dalquen P, Thiel G. [Phenacetin abuse. VII: Urinary
tract tumors in dialysis patients and patients with kidney grafts]. Schweiz Med Wochenschr 1982;
112(42): 1468-1472.
14
Orell SR, Nanra RS, Ferguson NW. Renal pelvic carcinoma in the Hunter Valley. Med J Aust 1979;
2(10): 524, 555-524, 557.
15
Porpaczy P, Schramek P. Analgesic nephropathy and phenacetin-induced transitional cell carcinoma analysis of 300 patients with long-term consumption of phenacetin-containing drugs. Eur Urol 1981;
7(6): 349-354.
16
Syre G, Matejka M. [Abuse of phenacetin-containing analgesics and carcinoma of the renal pelvis
(author's transl)]. Wien Klin Wochenschr 1981; 93(13): 420-423.
17
Anderstrom C, Johansson SL, Pettersson S, Wahlqvist L. Carcinoma of the ureter: a clinicopathologic
study of 49 cases. J Urol 1989; 142(2 Pt 1): 280-3.
18
Burnett KR, Miller JB, Greenbaum EI. Transitional cell carcinoma: rapid development in phenacetin
abuse. AJR Am J Roentgenol 1980; 134(6): 1259-61.
19
Holmäng SaJSL. Synchronous bilateral ureteral and renal pelvic carcinomas. Cancer 2004; 101(4):
741-747.
20
Lornoy W, Morelle V, Becaus I, Fonteyne E, Mestdagh J, Thienpont L et al. Malignant uroepithelial
tumors of the upper urinary tract in sixteen patients with analgesic nephropathy. Acta Clin Belg 1980;
35(3): 140-7.
21
Petersen I, Ohgaki H, Ludeke BI, Kleihues P. p53 Mutationen in Phenazetin-induzierten
Urothelkarzinomen. [p53 mutation in phenacetin-induced urothelial carcinomas]. Verh Dtsch Ges
Pathol 1993; 77: 252-5.
22
Steffens J, Nagel R. Tumours of the renal pelvis and ureter. Observations in 170 patients. Br J Urol
1988; 61(4): 277-83.
23
McCredie M, Ford JM, Taylor JS, Stewart JH. Analgesics and cancer of the renal pelvis in New
South Wales. Cancer 1982; 49(12): 2617-2625.
24
McCredie M, Stewart JH, Ford JM. Analgesics and tobacco as risk factors for cancer of the ureter and
renal pelvis. J Urol 1983; 130(1): 28-30.
25
McCredie M, Stewart JH, Ford JM, MacLennan RA. Phenacetin-containing analgesics and cancer of
the bladder or renal pelvis in women. Br J Urol 1983; 55(2): 220-224.
26
McLaughlin JK, Mandel JS, Blot WJ, Schuman LM, Mehl ES, Fraumeni JF, Jr. A population--based
case--control study of renal cell carcinoma. J Natl Cancer Inst 1984; 72(2): 275-284.
27
Piper JM, Tonascia J, Matanoski GM. Heavy phenacetin use and bladder cancer in women aged 20 to
49 years. N Engl J Med 1985; 313(5): 292-295.
34
Phenacetin
28
Piper JM, Matanoski GM, Tonascia J. Bladder cancer in young women. Am J Epidemiol 1986;
123(6): 1033-1042.
29
Castelao JE, Yuan JM, Gago DM, Yu MC, Ross RK. Non-steroidal anti-inflammatory drugs and
bladder cancer prevention. Br J Cancer 2000; 82(7): 1364-1369.
30
Fortuny J, Kogevinas M, Garcia-Closas M, Real FX, Tardon A, Garcia-Closas R et al. Use of
analgesics and nonsteroidal anti-inflammatory drugs, genetic predisposition, and bladder cancer risk
in Spain. Cancer Epidemiol Biomarkers Prev 2006; 15(9): 1696-1702.
31
Fortuny J, Kogevinas M, Zens MS, Schned A, Andrew AS, Heaney J et al. Analgesic and antiinflammatory drug use and risk of bladder cancer: a population based case control study. BMC Urol
2007; 7: 13.
32
Gago Dominguez M, Yuan JM, Castelao JE, Ross RK, Yu MC. Regular use of analgesics is a risk
factor for renal cell carcinoma. Br J Cancer 1999; 81(3): 542-8.
33
Jensen OM, Knudsen JB, Tomasson H, Sorensen BL. The Copenhagen case-control study of renal
pelvis and ureter cancer: role of analgesics. Int J Cancer 1989; 44(6): 965-8.
34
Kreiger N, Marrett LD, Dodds L, Hilditch S, Darlington GA. Risk factors for renal cell carcinoma:
results of a population-based case-control study. Cancer Causes Control 1993; 4(2): 101-110.
35
Linet MS, Chow WH, McLaughlin JK, Wacholder S, Yu MC, Schoenberg JB et al. Analgesics and
cancers of the renal pelvis and ureter. Int J Cancer 1995; 62(1): 15-8.
36
McCredie M, Stewart JH, Carter JJ, Turner J, Mahony JF. Phenacetin and papillary necrosis:
independent risk factors for renal pelvic cancer. Kidney Int 1986; 30(1): 81-4.
37
McCredie M, Stewart JH. Does paracetamol cause urothelial cancer or renal papillary necrosis?
Nephron 1988; 49(4): 296-300.
38
McCredie M, Coates MS, Ford JM, Disney AP, Auld JJ, Stewart JH. Geographical distribution of
cancers of the kidney and urinary tract and analgesic nephropathy in Australia and New Zealand.
Aust N Z J Med 1990; 20(5): 684-8.
39
McCredie M, Stewart JH, Day NE. Different roles for phenacetin and paracetamol in cancer of the
kidney and renal pelvis. Int J Cancer 1993; 53(2): 245-9.
40
McCredie M, Pommer W, McLaughlin JK, Stewart JH, Lindblad P, Mandel JS et al. International
renal-cell cancer study. II. Analgesics. Int J Cancer 1995; 60(3): 345-9.
41
McLaughlin JK, Blot WJ, Mehl ES, Fraumeni JF. Relation of analgesic use to renal cancer:
population-based findings. Natl Cancer Inst Monogr 1985; 69: 217-22.
42
McLaughlin JK, Gao YT, Gao RN, Zheng W, Ji BT, Blot WJ et al. Risk factors for renal-cell cancer
in Shanghai, China. Int J Cancer 1992; 52(4): 562-5.
43
Pommer W, Bronder E, Klimpel A, Helmert U, Greiser E, Molzahn M. Urothelial cancer at different
tumour sites: role of smoking and habitual intake of analgesics and laxatives. Results of the Berlin
Urothelial Cancer Study. Nephrol Dial Transplant 1999; 14(12): 2892-7.
44
Stewart JH, Hobbs JB, McCredie MR. Morphologic evidence that analgesic-induced kidney
pathology contributes to the progression of tumors of the renal pelvis. Cancer 1999; 86(8):
1576-1582.
References
35
45
Schmal D, REITER A. [Absence of carcinogenic effect in phenacetin.]. Arzneimittelforschung 1954;
4(6): 404-405.
46
Calder IC, Goss DE, Williams PJ, Funder CC, Green CR, Ham KN et al. Neoplasia in the rat induced
by N-hydroxyphenacetin, a metabolite of phenacetin. Pathology 1976; 8(1): 1-6.
47
Johansson S, Angervall L. Urothelial changes of the renal papillae in Sprague-Dawley rats induced
by long term feeding of phenacetin. Acta Pathol Microbiol Scand [A] 1976; 84(5): 375-383.
48
Isaka H, Yoshii H, Otsuji A, Koike M, Nagai Y, Koura M et al. Tumors of Sprague-Dawley rats
induced by long-term feeding of phenacetin. Gann 1979; 70(1): 29-36.
49
Nakanishi K, Kurata Y, Oshima M, Fukushima S, Ito N. Carcinogenicity of phenacetin: long-term
feeding study in B6c3f1 mice. Int J Cancer 1982; 29(4): 439-44.
50
Johansson SL, Radio SJ, Saidi J, Sakata T. The effects of acetaminophen, antipyrine and phenacetin
on rat urothelial cell proliferation. Carcinogenesis 1989; 10(1): 105-11.
51
Bogdanffy MS, Mazaika TJ, Fasano WJ. Early cell proliferative and cytotoxic effects of phenacetin
on rat nasal mucosa. Toxicol Appl Pharmacol 1989; 98(1): 100-12.
52
Usui T, Mutai M, Hisada S, Takoaka M, Soper KA, McCullough B et al. CB6F1-rasH2 mouse:
overview of available data. Toxicol Pathol 2001; 29 Suppl: 90-108.
53
Storer RD, French JE, Haseman J, Hajian G, LeGrand EK, Long GG et al. P53+/- hemizygous
knockout mouse: overview of available data. Toxicol Pathol 2001; 29 Suppl: 30-50.
54
Eastin WC, Mennear JH, Tennant RW, Stoll RE, Branstetter DG, Bucher JR et al. Tg.AC genetically
altered mouse: assay working group overview of available data. Toxicol Pathol 2001; 29 Suppl: 6080.
55
Lina BA, Woutersen RA, Bruijntjes JP, van Benthem J, van den Berg JA, Monbaliu J et al. Evaluation
of the Xpa-deficient transgenic mouse model for short-term carcinogenicity testing: 9-month studies
with haloperidol, reserpine, phenacetin, and D-mannitol. Toxicol Pathol 2004; 32(2): 192-201.
56
Patierno SR, Lehman NL, Henderson BE, Landolph JR. Study of the ability of phenacetin,
acetaminophen, and aspirin to induce cytotoxicity, mutation, and morphological transformation in
C3H/10T1/2 clone 8 mouse embryo cells. Cancer Res 1989; 49(4): 1038-44.
57
Mauthe RJ, Gibson DP, Bunch RT, Custer L. The syrian hamster embryo (SHE) cell transformation
assay: review of the methods and results. Toxicol Pathol 2001; 29 Suppl: 138-46.
58
Shibata MA, Sano M, Hagiwara A, Hasegawa R, Shirai T. Modification by analgesics of lesion
development in the urinary tract and various other organs of rats pretreated with dihydroxy-di-Npropylnitrosamine and uracil. Jpn J Cancer Res 1995; 86(2): 160-7.
59
Tanooka H. Development and applications of Bacillus subtilis test systems for mutagens, involving
DNA-repair deficiency and suppressible auxotrophic mutations. Mutat Res 1977; 42(1): 19-31.
60
Shudo K, Ohta T, Orihara Y, Okamoto T, Nagao M, Takahashi Y et al. Mutagenicities of phenacetin
and its metabolites. Mutat Res 1978; 58(2-3): 367-370.
61
King MT, Beikirch H, Eckhardt K, Gocke E, Wild D. Mutagenicity studies with x-ray-contrast media,
analgesics, antipyretics, antirheumatics and some other pharmaceutical drugs in bacterial, Drosophila
and mammalian test systems. Mutat Res 1979; 66(1): 33-43.
36
Phenacetin
62
Camus AM, Friesen M, Croisy A, Bartsch H. Species-specific activation of phenacetin into bacterial
mutagens by hamster liver enzymes and identification of N-hydroxyphenacetin O-glucuronide as a
promutagen in the urine. Cancer Res 1982; 42(8): 3201-8.
63
Dunkel VC, Simmon VF. Mutagenic activity of chemicals previously tested for carcinogenicity in the
National Cancer Institute bioassay program. IARC Sci Publ 1980;(27): 283-301.
64
Weinstein D, Katz M, Kazmer S. Use of rat/hamster S-9 mixture in the Ames mutagenicity assay.
Environ Mutagen 1981; 3(1): 1-9.
65
De Flora S, Russo P, Pala M, Fassina G, Zunino A, Bennicelli C et al. Assay of phenacetin
genotoxicity using in vitro and in vivo test systems. J Toxicol Environ Health 1985; 16(3-4): 355-77.
66
Oldham JW, Preston RF, Paulson JD. Mutagenicity testing of selected analgesics in Ames Salmonella
strains. J Appl Toxicol 1986; 6(4): 237-43.
67
Fassina G, ABBONDANDOLO A, MARIANI L, TANINGHER M, Parodi S. Mutagenicity in V79
cells does not correlate with carcinogenicity in small rodents for 12 aromatic amines. J Tox Env
Health 1990; 29(1): 109-130.
68
Luijten M, Speksnijder EN, van Alphen N, Westerman A, Heisterkamp SH, van Benthem J et al.
Phenacetin acts as a weak genotoxic compound preferentially in the kidney of DNA repair deficient
Xpa mice. Mutat Res 2006; 596(1-2): 143-150.
69
Adams SP, Laws GM, Storer RD, DeLuca JG, Nichols WW. Detection of DNA damage induced by
human carcinogens in acellular assays: Potential application for determining genotoxic mechanisms.
Mutat Res ; 1996; 368(3-4): 235-248.
70
Granberg Ohman I, Johansson S, Hjerpe A. Sister-chromatid exchanges and chromosomal
aberrations in rats treated with phenacetin, phenazone and caffeine. Mutat Res 1980; 79(1): 13-8.
71
Sutou S, Kondo M, Mitsui Y. Effects of multiple dosing of phenacetin in the micronucleus test. Mutat
Res 1990; 234(3-4): 183-6.
72
Sutou S, Mitui Y, Toda S, Sekijima M, Kawasaki K, Ando N et al. Effect of multiple dosing of
phenacetin on micronucleus induction: a supplement to the international and Japanese cooperative
studies. Mutat Res 1990; 245(1): 11-4.
73
Sicardi SM, Martiarena JL, Iglesias MT. Mutagenic and analgesic activities of aniline derivatives. J
Pharm Sci ; 1991; 80(8): 761-764.
74
Higashikuni N, Baba T, Nakamura T, Sutou S. The micronucleus test with peripheral reticulocytes
from phenacetin-treated mice. Mutat Res 1992; 278(2-3): 159-64.
75
Asanami S, Shimono K, Sawamoto O, Kurisu K, Uejima M. The suitability of rat peripheral blood in
subchronic studies for the micronucleus assay. Mutat Res 1995; 347(2): 73-8.
76
Holme JA, Soderlund E. Species differences in cytotoxic and genotoxic effects of phenacetin and
paracetamol in primary monolayer cultures of hepatocytes. Mutat Res 1986; 164(3): 167-75.
77
Gotoh S, Higashi K, Miyata Y, Nishi C, Sakamoto Y. Screening for carcinogens by DNA-synthesis
inhibition test using mouse L-cells. J UOEH 1983; 5(2): 147-53.
References
37
78
Robbiano L, Allavena A, Bagarolo C, Martelli A, Brambilla G. Comparison in human and rat
hepatocytes of the DNA-damaging activity of five chemicals probably carcinogenic to humans.
Toxicol in Vitro; 1994; 8(1): 131-137.
79
Guideline to the classification of carcinogenic compounds. Health Council of The Netherlands,
editor. The Hague, The Netherlands: 2010: publication no. A10/07E.
38
Phenacetin
A
Request for advice
B
The Committee
C
The submission letter
D
Comments on the public review draft
E
IARC Monograph
F
Human data
G
Animal data
H
Genotoxicity data
I
Carcinogenic classification of substances by the Committee
Annexes
39
40
Phenacetin
Annex
A
Request for advice
In a letter dated October 11, 1993, ref DGA/G/TOS/93/07732A, to, the State
Secretary of Welfare, Health and Cultural Affairs, the Minister of Social Affairs
and Employment wrote:
Some time ago a policy proposal has been formulated, as part of the simplification of the governmental advisory structure, to improve the integration of the development of recommendations for health
based occupation standards and the development of comparable standards for the general population.
A consequence of this policy proposal is the initiative to transfer the activities of the Dutch Expert
Committee on Occupational Standards (DECOS) to the Health Council. DECOS has been established
by ministerial decree of 2 June 1976. Its primary task is to recommend health based occupational
exposure limits as the first step in the process of establishing Maximal Accepted Concentrations
(MAC-values) for substances at the work place.
In an addendum, the Minister detailed his request to the Health Council as
follows:
The Health Council should advice the Minister of Social Affairs and Employment on the hygienic
aspects of his policy to protect workers against exposure to chemicals. Primarily, the Council should
report on health based recommended exposure limits as a basis for (regulatory) exposure limits for air
quality at the work place. This implies:
•
A scientific evaluation of all relevant data on the health effects of exposure to substances using a
criteria-document that will be made available to the Health Council as part of a specific request
Request for advice
41
for advice. If possible this evaluation should lead to a health based recommended exposure limit,
or, in the case of genotoxic carcinogens, a ‘exposure versus tumour incidence range’ and a
calculated concentration in air corresponding with reference tumour incidences of 10-4 and 10-6
per year.
•
The evaluation of documents review the basis of occupational exposure limits that have been
recently established in other countries.
•
Recommending classifications for substances as part of the occupational hygiene policy of the
government. In any case this regards the list of carcinogenic substances, for which the
classification criteria of the Directive of the European Communities of 27 June 1967 (67/548/
EEG) are used.
•
Reporting on other subjects that will be specified at a later date.
In his letter of 14 December 1993, ref U 6102/WP/MK/459, to the Minister of
Social Affairs and Employment the President of the Health Council agreed to
establish DECOS as a Committee of the Health Council. The membership of the
Committee is given in Annex B.
42
Phenacetin
Annex
B
The Committee
•
•
•
•
•
•
•
•
R.A. Woutersen, chairman
Toxicologic Pathologist, TNO Innovation for Life, Zeist; Professor of
Translational Toxicology, Wageningen University and Research Centre,
Wageningen
J. van Benthem
Genetic Toxicologist, National Institute for Public Health and the
Environment, Bilthoven
P.J. Boogaard
Toxicologist, SHELL International BV, The Hague
G.J. Mulder
Emeritus Professor of Toxicology, Leiden University, Leiden
Ms M.J.M. Nivard
Molecular Biologist and Genetic Toxicologist, Leiden University Medical
Center, Leiden
G.M.H. Swaen
Epidemiologist, Dow Chemicals NV, Terneuzen
E.J.J. van Zoelen
Professor of Cell Biology, Radboud University Nijmegen, Nijmegen
G.B. van der Voet, scientific secretary
Health Council of the Netherlands, The Hague
The Committee
43
The Health Council and interests
Members of Health Council Committees are appointed in a personal capacity
because of their special expertise in the matters to be addressed. Nonetheless, it
is precisely because of this expertise that they may also have interests. This in
itself does not necessarily present an obstacle for membership of a Health
Council Committee. Transparency regarding possible conflicts of interest is
nonetheless important, both for the chairperson and members of a Committee
and for the President of the Health Council. On being invited to join a
Committee, members are asked to submit a form detailing the functions they
hold and any other material and immaterial interests which could be relevant for
the Committee’s work. It is the responsibility of the President of the Health
Council to assess whether the interests indicated constitute grounds for nonappointment. An advisorship will then sometimes make it possible to exploit the
expertise of the specialist involved. During the inaugural meeting the
declarations issued are discussed, so that all members of the Committee are
aware of each other’s possible interests.
44
Phenacetin
Annex
C
The submission letter
Subject
Our reference
Your Reference
Enclosed
Date
: Submission of the advisory report Phenacetin
: U-7412/BvdV/fs/246-C17
: DGV/MBO/U-932342
:1
: November 13, 2012
Dear State Secretary,
I hereby submit the advisory report on the effects of occupational exposure to
Phenacetin.
This advisory report is part of an extensive series in which carcinogenic
substances are classified in accordance with European Union guidelines. This
involves substances to which people can be exposed while pursuing their
occupation.
The advisory report was prepared by the Subcommittee on the Classification
of Carcinogenic Substances, a permanent subcommittee of the Health Council’s
Dutch Expert Committee on Occupational Safety (DECOS). The advisory report
has been assessed by the Health Council’s Standing Committee on Health and
the Environment.
The submission letter
45
I have today sent copies of this advisory report to the State Secretary of
Infrastructure and the Environment and to the Minister of Health, Welfare and
Sport, for their consideration.
Yours sincerely,
(signed)
Professor W.A. van Gool
President
46
Phenacetin
Annex
D
Comments on the public review draft
A draft of the present report was released in June 2012 for public review. No
comments were received on the draft document.
Comments on the public review draft
47
48
Phenacetin
Annex
E
IARC Monograph
Volume 100A, 2011 (excerpt from Phenacetin, pp397-400)
Phenacetin was considered by previous IARC Working Groups in 1976 and
1980. Analgesic mixtures containing phenacetin were considered by a previous
IARC Working Group in 1987. Since that time, new data have become available,
these have been incorporated in the Monograph, and taken into consideration in
the present evaluation.
5
Evaluation
There is sufficient evidence in humans for the carcinogenicity of analgesic
mixtures containing phenacetin. Analgesic mixtures containing phenacetin cause
cancer of the renal pelvis, and of the ureter.
There is limited evidence in experimental animals for the carcinogenicity of
analgesic mixtures containing phenacetin.
There is sufficient evidence in humans for the carcinogenicity of phenacetin.
Phenacetin causes cancer of the renal pelvis, and of the ureter.
There is sufficient evidence in experimental animals for the carcinogenicity
of phenacetin.
Analgesic mixtures containing phenacetin are carcinogenic to humans
(Group 1).
Phenacetin is carcinogenic to humans (Group 1).
IARC Monograph
49
For the overall evaluation of phenacetin, the Working Group took into
consideration that tumours of the renal pelvis and ureter are not known to result
from the other components of the analgesic mixtures used in most countries;
namely, aspirin, codeine phosphate, and caffeine.
50
Phenacetin
Annex
F
Human data
Human case-control studies of phenacetin exposure and different forms of cancer (published after the IARC publication of
1987).
reference design/population
results
confounding
remarks
factors
risk ratio(95% CI)
exposure
cases /
phenacetin
controla
containing drugs
renal pelvic cancer
McLaugh population-based case- never
m 24/232
OR 1
adjusted for age the separate effects
lin et al., control, Minneapolis,
f 12/147
OR 1
and cigarette
of the analgesics
198541
ever
m 26/196
OR 1.2 (0.6-2.4)
smoking.
could not be
US
adequately
f 9/100
OR 1.3 (0.5-3.4)
OR 1.1 (0.6-2.3)
assessed because
(74 cases and 697
irregular
m 21/175
f 12/122
OR 1.1 (0.4-3.2)
most long-term
controls, identified
regular ≤36 mo m 1/17
OR 0.5 (0.02-3.9)
users took both
between 1974-1979)
phenacetin and
f 1/12
OR 1.8 (0.4-22.0)
regular>36 mo m 4/4
OR 8.1 (1.2-62.2)
acetaminophenf 2/10
OR 4.2 (0.4-42.0)
containing
products
McCredie hospital-based case32/672
adjusted for sex
no consumption
et al.,
control, Sidney, New
(lifetime exposure
198636
South Wales, Australia < 1kg)
(66 cases and 751
lifetime exposure > 27/35
RR 20 (12-34)
controls, identified
1 kg with RPN
between 1970-1982)
lifetime exposure > 7/44
RR 3.6 (1.6-8.1)
1 kg absence of
RPN
Human data
51
≥ 1 kg / lifetime
33/54
OR 7.9 (4.6-13.8)
> 0.1 kg / lifetime
40/636
OR 5.7 (3.2-10.0)
non-consumers
< 2.04 kg/ lifetime
2.04-6.87 kg/
lifetime
> 6.88 kg/ lifetime
consumption of
aspirin or
phenacetin
Stewart et “blinded”
< 1 kg / lifetime
al.,
histopathological
1.0-4.9 kg /
199944
review of cases from
lifetime
population- based case- 5.0-9.9 kg /
control study, New
lifetime
South Wales, Australia ≥ 10.0 kg / lifetime
76/474
12/16
16/16
OR 1
OR 5.2 (2.2-12.4)
OR 8.3 (3.4-20.5)
42/17
OR 18.5 (8.7-39.9)
20/37
6/5
RR 1.0
RR 1.9 (0.5-7.3)
5/4
RR 2.1 (0.5-8.9)
17/5
RR 5.6 (1.8-18)
Pommer
et al.,
199943
no/rare analgesic
intake
> 1.0 kg / lifetime
20/19
OR 1.0
7/2
OR 5.3 (0.3-81)
adjusted never
used
ever used
31/113
9/55
13/12
17/15
31/113
9/55
13/12
17/15
6/7
2/3
5/2
7/7
4/4
6/4
385/369
21/23
9/12
RR 1.0
RR 1.0
RR 2.4 (0.9-6.8)
RR 4.2 (1.5-12.3)
RR 1.0
RR 1.0
RR 3.9 (1.7-9.1)
RR 6.9 (2.7-17.7)
RR 3.1 (1.0-9.6)
RR 6.1 (1.5-25.6)
RR 9.1 (2.2-38)
RR 6.1 (1.9-20.0)
RR 2.4 (0.4-14.5)
RR 9.2 (2.5-33)
OR 1.0
OR 0.8 (0.4-1.6)
OR 0.3 (0.3-2.1)
McCredie population-based caseet al.,
control, New South
198837
Wales, Australia
(73 cases and 688
controls, identified
between 1980-1982)
McCredie population-based caseet al.,
control, New South
199339
Wales, Australia
(147 cases and 523
controls identified in
1989-1990)
hospital-based and
population-based
case-control, (former)
West Berlin, Germany
(51 cases and 647
controls)
Jensen et hospital-based
al.,
case-control,
198933
Copenhagen, the island
of Sjaelland, Denmark
(96 cases and 294
controls, identified
between 1979 and
1982)
crude: never
used
ever used
1-749 g
> 750 g
dose unknown
Linet et
al.,
199535
52
m
f
m
f
m
f
m
f
m
f
m
f
m
f
population-based
no regular use
case-control, New
≤ 1.0 kg / lifetime
Jersey, Iowa and Los
> 1.0 kg / lifetime
Angeles, US (502 cases
and 496 controls
identified between
1983-1986)
Phenacetin
adjusted for sex most cases were
and exposure to included in
paracetamol and previous studies
tobacco
adjusted for age,
sex method of
interview,
cigarette
smoking,
paracetamol in
any form and
educational level
adjusted for age this study used the
and smoking
same cases as
McCredie et al.,
1993
adjusted for
socioeconomic
status, cigarette
smoking and
laxative intake
adjusted for age,
sex, tobacco
smoking and
occupational
exposures
known to be
associated with
high risks of
these cancers
79% of the tumours
were located in the
renal pelvis
including calyces
adjusted for age,
sex, geographic
area and
cigarette
smoking
308 cases with
renal pelvis cancer
and 194 cases with
ureter cancer
This study only
contained small
number of regular
analgesic users and
no analgesic
abusers.
Pommer
et al.,
199943
hospital-based and
population-based
case-control, West
Berlin, Germany (76
cases and 647 controls)
ureter cancer
McCredie population-based caseet al.,
control, New South
198837
Wales, Australia
(55 cases and 688
controls, identified
between 1980-1982)
renal cell cancer
McLaugh population-based caselin et al., control, Minneapolis,
198541
US
(495 cases and 697
controls, identified
between 1974-1979)
> 1.0 kg / lifetime
7/3
OR 1.8 (0.2-13)
adjusted for
51 cases with renal
socioeconomic pelvis and 25 cases
status, cigarette with ureter cancer
smoking and
laxative intake.
≥ 1 kg / lifetime
> 0.1 kg / lifetime
6/54
49/636
OR 1.2 (0.5-3.0)
OR 0.7 (0.3-2.2)
adjusted for sex
and exposure to
paracetamol and
tobacco
never
188/232
74/147
125/196
108/122
99/175
86/100
18/17
10/12
8/4
12/10
OR 1.0
OR 1.0
OR 0.7 (0.5-1.0)
OR 1.7 (1.1-2.7)
OR 0.7 (0.5-0.9)
OR 1.7 (1.1-2.6)
OR 1.3 (0.6-2.7)
OR 1.9 (0.7-5.6)
OR 2.2 (0.6-8.9)
OR 2.4 (0.8-6.7)
adjusted for age
and cigarette
smoking.
McCredie hospital-based caseet al.,
control, Sidney, New
198636
South Wales, Australia
(86 cases and 751
controls, identified
between 1970-1982)
72/672
no consumption
(lifetime exposure
< 1kg)
lifetime exposure > 1/35
1 kg with RPN
m
f
m
ever
f
m
irregular
f
regular ≤36 mo m
f
regular>36 mo m
f
lifetime exposure > 13/44
1 kg absence of
RPN
McLaugh population-based case- regular use (at least 154/157
lin et al., control, Shanghai,
2 times/week for 2
198541
China (154 cases and
weeks or longer)
157 controls, identified
between 1978-1989)
McCredie population-based caseet al.,
control, New South
199339
Wales, Australia
(489 cases and 523
controls identified in
1989-1990)
Human data
non-consumers
< 2.04 kg/ lifetime
2.04-6.87 kg/
lifetime
> 6.88 kg/ lifetime
consumption of
aspirin or
phenacetin
adjusted for sex
RR 2.5 (1.3-4.9)
RR 0.4 ( 0.1-2.7)
OR 2.3 (0.7-7.0)
adjusted for age,
sex, education,
BMI and
cigarette
smoking.
420/474
21/16
24/16
OR 1
OR 1.4 (0.7-2.9)
OR 1.8 (0.9-3.5)
17/17
OR 1.0 (0.5-2.1)
adjusted for age,
sex method of
interview,
cigarette
smoking,
paracetamol in
any form and
obesity
53
Kreiger et population-based caseal.,
control, Ontario,
199334
Canada (490 cases and
1351 controls,
identified between
1986-1987)
no phenacetin or m 265/578
acetaminophen f 166/580
phenacetin only m 2/2
f 3/7
phenacetin and m 3/4
acetaminophen f 0/8
any phenacetin m 5/6
f 3/15
McCredie case-control, data
reference group m 839/1094
f 474/630
et al.,
pooled from studies in
199540
m 14/28
Australia, Denmark,
< 0.1 kg
f 17/22
Germany, Sweden and
> 0.1 kg
m 46/67
US (1313 cases and
1724 controls,
f 51/58
identified between
0.1-1.0 kg
m 25/48
1989-1991)
f 26/32
1.1-5.0 kg
m 16/17
f 20/14
> 5 kg
m 5/2
f 5/12
Gagopopulation-based case non/irregular use 616/744
Domin- control, Los Angeles,
analgesics
guez et
California, US (1204
regular use
86/55
al.,
cases and 1204 controls, max weekly dose 41/37
199932
identified between
<2 g
1986-1994)
max weekly dose 22/6
2-<4 g
max weekly dose 23/12
4-<8 g
bladder cancer
McCredie population-based caseet al.,
control, New South
198837
Wales, Australia
(162 cases and 688
controls, identified
between 1980-1982)
Pommer et hospital-based and
al., 199943 population-based
case-control, (former)
West Berlin, Germany
(571 cases and 647
controls, identified
between 1990-1994)
Castelao et population-based caseal., 200029 control, Los Angeles,
USA
(1514 cases and 1514
controls,
1987-1996)
54
Phenacetin
OR 1.0
OR 1.0
OR 2.5 (0.3-18.5)
OR 1.8 (0.5-7.3)
OR 1.4 (0.3-6.7)
OR 1.7 (0.5-5.9)
OR 0.8 (0.2-2.7)
RR 1.0
RR 1.0
RR 0.6 (0.3-1.2)
RR 1.1 (0.6-2.3)
RR 0.9 (0.6-1.4)
RR 1.4 (0.9-2.1)
RR 0.7 (0.4-1.2)
RR 1.3 (0.7-2.3)
RR 1.3 (0.6-2.7)
RR 2.1 (1.0-4.4)
RR 2.6 (0.5-14.2)
RR 0.6 (0.2-1.8)
OR 1.0
OR 1.9 (1.3-2.7)
OR 1.3 (0.8-2.2)
OR 4.1 (1.5-10.8)
OR 2.3 (1.0-5.0)
adjusted for age,
active cigarette
smoking and
combined
Quetelet index
this study included
only a small
amount of
phenacetin users
adjusted for
centre, age, sex,
BMI, cigarette
smoking
the RR as not
changed by
additional
adjustment for
consumption of
paracetamol or
other analides
this study only
contained a small
number of regular
analgesics users
and the amount of
consumed
analgesics was also
small
adjusted for
level of
education, BMI,
cigarette
smoking,
hypertension,
use
amphetamines.
≥ 1 kg / lifetime 27/54
≥ 0.1 kg / lifetime 135/636
OR 2.0 (1.1-3.5)
OR 2.1 (1.3-3.5)
adjusted for sex most cases were
and exposure to included in previous
paracetamol and studies
tobacco
> 1.0 kg / lifetime 23/23
OR 0.7 (0.4-1.4)
adjusted for
socioeconomic
status, cigarette
smoking and
laxative intake.
non/irregular use
analgesics
regular use
< 46 g / lifetime
46-250 g / lifetime
>250 g / lifetime
961/920
OR 1.0
82/64
25/18
27/20
21/20
OR 1.5 (0.9-2.7)
OR 1.4 (0.6-3.1)
OR 1.6 (0.7-3.7)
OR 1.9 (0.8-4.4)
adjusted for level
of education,
cigarette
smoking, NSAID
use, use other
analgesics,
employment as
hairdresser
Fortuny et hospital-based caseal., 200630 control, Spain (958 case
and 1029 controls,
identified between
1997-2000)
nonusers
848/893
ever use
59/67
52/55
non regular use
(> 20 times lifelong
and < 2 times/week
for 1 month)
regular use (> 2
7/12
times/week for ≥ 1
month)
Fortuny et population-based case never use
313/421
al., 200731 control, New
ever use
53/35
Hampshire, UK (376
duration 4 yr
22/14
cases and 463 controls, duration 4-8 yr
6/9
identified between
duration > 8 yr
25/12
1998-2001)
a
OR 1.0
OR 1.1 (0.7-2.0)
OR 1.1 (0.6-2.0)
adjusted for age,
sex, region,
cigarette
smoking, use
other NSAID or
analgesics
OR 1.3 (0.3-4.5)
OR 1.0
OR 2.2 (1.3-3.8)
OR 2.2 (1.0-4.7)
OR 1.1 (0.4-3.5)
OR 3.0 (1.4-6.5)
adjusted for age,
sex, region,
cigarette
smoking, use
other NSAID or
analgesics
The number of cases and controls do not necessarily add up to the total number of cases and controls of the whole study (as
mentioned in the second column), since in many studies also exposure to other (non-phenacetin-containing) analgesics are
studied.
Human data
55
56
Phenacetin
Annex
G
Animal data
animal species,
(number, sex, age)
RAT, BD I & III
30, sex unspecified,
100 d
RAT, albino, 15-24,
male
dose, route of exposure
duration
carcinogenic effects
ref.
40-50 mg phenacetin oral
(diet) (average total dose,
22 g)
0.05, 0.1 or 0.5 % Nhydroxyphenacetin oral
(diet)
0.535% phenacetin oral
(diet)
2 yr
no tumours observed
2
1.5 yr
hepatocellular carcinomas
2
1.5-2 y
carcinomas of the mammary gland and ear duct
3
RAT, S-D, 50 male, 1.25-2.5% phenacetin oral
50 female, 9 wk
(diet)
1.5 yr
3
MICE, B6C3F1,
52 m+f,
RAT, S-D, 20, m,
6 wk
RAT, F344,
10-20, m, 6 wk
2 yr
tumours in nasal cavity
tumours in the urinary tract
papillomas (only in female)
renal cell adenoma
kidney, liver, lung, skin and hemapotopoietic tumours
increased labeling index kidney and bladder
RAT, S-D, female
RAT, Crl:CDBR
0.6-1.25 % phenacetin oral
(diet)
0.05, 1.0 and 1.5% oral
(diet)
pretreatment 0.1% DHPN
and 3.0% uracil
+phenacetin 2.0% oral
(diet) (1068-1145 mg/kg/d)
100, 625 and 1250 mg/kg,
oral (gavage)
Animal data
6-12 wk
1
50
35 wk
renal cell tumours in the pre-treated rats.
no tumours in the non pre-treated rats.
58
7-14 d
increased DNA synthesis in respiratory and olfactory
mucosa
51
57
58
Phenacetin
Annex
H
Genotoxicity data
In vitro assays.
test
cell line/species
concentration
results
0.2-2.5 mM
0.1010 mM
0.25-2.5 mM
sublethal doses
<10 mg/plate
NT
-
- act.
DNA fragmentation
Calf thymus DNA
λ DNA
λ DNA
gene mutation test in
S.thyphimurium
bacteria
TA97, TA98, TA100
reverse mutation test
and TA102
gene mutation test in
TA98, TA 100,
bacteria
TA1,535, TA1,537,
reverse mutation test
TA1,538
DNA-repair test
E.coli strains:
WP2uvrA, WP67,
TM1,080, TM1,080
DNA synthesis inhibition mouse L-cells
test
alkaline elution assay
rat hepatocytes
human
hepatocytes
unscheduled DNA
liversynthesis (UDS) test
hepatocytes, mouse,
rat, guina pig or
hamster
Genotoxicity data
remarks
reference
+ act.
Adams et al.,
199669
NT
+
+ TA100
De Flora
et al., 198565
5,50,500, 1,000, 2,500 and 5,000 µg/plate
+ TA100
Oldham et
al., 198666
0.3, 1, 3 mg/plate
-
NT
De Flora et
al., 198565
1 mM
NT
0, 1, 1.8, 3.2 mM
0, 1, 1.8, 3.2 mM
-
+
(rat-S9)
NT
NT
Goto et al.,
198377
Robbiano et
al., 199478
0.1, 0.5, 1, 2.5, 5,
10 mM, 18-19 h
-
UDS measured
by scintillation
counting
Holme et al.,
198676
59
gene mutation test in
mammalian cells
Hprt-test
V79
0, 1 and 5 mM
-
gene mutation test in
mammalian cells
Hprt-test
V79
0, 1, 1.5, 5, 7.5 mM
-
(rat-S9)
±
(hamster-S9)
(rat-S9)
+
(hamster-S9)
De Flora
et al., 198565
Fassina et
al., 199067
In vivo mutation assays.
test
species
alkaline elution assay
rat, liver cells
rat, kidney cells
route of
administration
i.p
micronucleus test in
peripheral blood cells
MS/Ae mice
330 mg/kg
gavage
+
i.p
i.p
i.p
i.p
i.p
330 mg/kg
37.5, 75, 150, 300, 400
and 600 mg/kg; 1, 2, 3
or 4 times
0, 2, 5, 50, 100 mg/kg,
1 dose
400, 600, 800 mg/kg
400, 600, 800 mg/kg
400, 600, 800 + 300,
400, 600, 800 mg/kg
400, 600 mg/kg
i.p
600 mg/kg
i.p
micronucleus test in
peripheral blood cells
Sprague-Dawley
rats
micronucleus test in bone
marrow cells
in vivo gen- mutation
C57BL/6 mice
assay with lacZ
transgenic mice
60
Phenacetin
gavage
oral, in
feed
remarks
+
-
i.p
micronucleus test in bone CD-1 mice
marrow cells
results
gavage
mouse, bone marrow i.p
sister chromatid
mouse
i.p
exchange test (SCE)
micronucleus test in bone CD-1 mice
i.p
marrow cells
micronucleus test in bone SJL Swiss mice
marrow cells
micronucleus test in
CD-1 mice
peripheral blood cells
dose
De Flora et
al., 198565
+
De Flora et
al., 198565
Sutou et al.,
199071
+
+
+
+
Sicardi et al.,
199173
single treatment Higashikuni
double treatment et al., 199274
+
single treatment
+
double treatment
+
single treatment
+
double treatment
500, 1,000, 2,000
mg/ml during 2 days
+
250, 500, 750, 1,000
mg/ml during 14 days
+
250, 500, 750, 1,000
mg/ml during 14 days
0.75% w/w, 4, 8 and
12 weeks
+
+
reference
Asanami et
al., 199575
sample times on
day 1,3,6,9,12
and 15.
sample times 4, 8 Luijten et al.,
or 12 weeks
200668
Annex
I
Carcinogenic classification of
substances by the Committee
The Committee expresses its conclusions in the form of standard phrases:
Category
Judgement of the Committee (GRGHS)
1A
The compound is known to be carcinogenic to humans.
• It acts by a stochastic genotoxic mechanism.
• It acts by a non-stochastic genotoxic mechanism.
• It acts by a non-genotoxic mechanism.
• Its potential genotoxicity has been insufficiently investigated.
Therefore, it is unclear whether the compound is genotoxic.
The compound is presumed to be carcinogenic to humans.
• It acts by a stochastic genotoxic mechanism.
• It acts by a non-stochastic genotoxic mechanism.
• It acts by a non-genotoxic mechanism.
• Its potential genotoxicity has been insufficiently investigated.
Therefore, it is unclear whether the compound is genotoxic.
The compound is suspected to be carcinogenic to man.
The available data are insufficient to evaluate the carcinogenic
properties of the compound.
The compound is probably not carcinogenic to man.
1B
2
(3)
(4)
Comparable with EU Category
67/548/EEC
EC No 1272/2008
before
as from
12/16/2008
12/16/2008
1
1A
2
1B
3
not applicable
2
not applicable
not applicable
not applicable
Source: Health Council of the Netherlands. Guideline to the classification of carcinogenic compounds. The Hague: Health
Council of the Netherlands, 2010; publication no. A10/07E.79
Carcinogenic classification of substances by the Committee
61
62
Phenacetin