Word file (43 KB )
advertisement

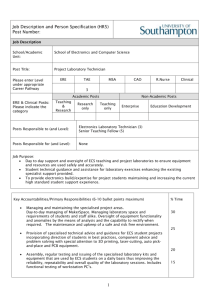
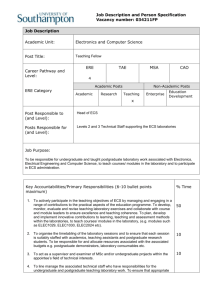
Supplementary information Characterization of DN SEMA3 receptors - Vertebrate class 3 semaphorins (SEMA3 proteins) consist of six (SEMA3A to SEMA3F) secreted disulfide-bound homodimeric molecules. Neuropilin-1 (Npn-1) and Npn-2 are vertebrate transmembrane glycoproteins acting as key components of SEMA3 receptors. The extracellular moiety of Npn contains two repeated complement-binding domains (CUB domains, a1-a2 domains), two coagulation factor-like domains (b1-b2 domains) and a juxtamembrane meprin/A5/muphosphatase (MAM, c) homology domain. The intracellular moiety contains a transmembrane and a small cytoplasmic domain without signalling motifs. Npn-1 and Npn-2 form ligand independent homo- and heterodimers. SEMA3 proteins elicit Npn noncovalent oligomerization, which is absolutely required for biological response1-4. Npn-1 homodimers confer responsiveness to SEMA3A and SEMA3E, Npn-2 homodimers to SEMA3F, Npn-1/Npn-2 heterodimers to SEMA3B and SEMA3C 5. The a1-a2 and b1-b2 domains of Npn bind respectively sema and Ig-basic domains of SEMA3 proteins respectively, contributing to the specificity of the receptor3. The MAM/c domain is pivotal for Npn oligomerization in response to SEMA3 proteins and biological activity2,3. Vascular endothelial growth factor-A165 (VEGF-A165) interacts with b1-b2 domains of Npn-1 3,6-8 and competes with SEMA3 proteins for binding to Npn-19. Placental growth factor 2 (PlGF2) binds with strong affinity b1-b2 domains of Npn-1 as well. However, while VEGF-A165 signals through both VEGF-R1 and –R2, PlGF2 transduces only through the latter7,10,11. Besides Npn, additional component(s) are required to form functional receptor complexes for SEMA3 proteins. The plexins (Plex) are a family of 9 integral membrane proteins with a large cytoplasmic sex-plexin (SP) domain with homology to Ras GTPase activating proteins and interacting with Rhofamily GTPases 12 . While they are functional receptors for classes 1, 4, and 7 SEMA, Plex do not bind directly to SEMA3 proteins and constitute a signalling moiety in 1 SEMA3 receptor complex, while Npn provide the ligand binding sites. Npn-1/Plex-A1, Npn-1/Plex-A2, and Npn-1/Plex-A3 complexes transduce SEMA3A, Npn-2/Plex-A1 complex transduces SEMA3F, Npn-1/Plex-A2 and Npn-2/Plex-A2 complexes transduce SEMA3C 13. Deleting the cytoplasmic domain of Plex-A1 gives rise to a Plex-DN 14 blocks the response to both SEMA3A and SEMA3F (Fig. S2, a), but not to VEGF-A165 and PlGF2 (Fig. S2, b-d). We sought to generate a Npn DN construct which is capable of inhibiting the response to both SEMA3A and SEMA3F, but not VEGF-A165 and PlGF2. We found that a modified Npn-1 consisting of the MAM/c, transmembrane, and cytoplasmic domains (Fig. S1 a, b) behaves as a Npn-1-DN negative construct interfering with the biological response to both Npn-1 and Npn-2 binding SEMA3 proteins (Fig. S2, a), but not to VEGFA-165 and PlGF2 (Fig. S2, b-c). Such a DN activity results from an impaired homo- and hetero-oligomerization of Npn-1 and Npn-2 as well as from an inhibition of hetero-oligomerization between Npn-1 or Npn-2 and PlexA1 (Fig. S1 c). Since ligand induced association of Npn-1 with VEGF-R2 is the molecular basis by which Npn-1 enhances EC biological response to VEGF-A165 15 , we tested whether Npn-1-DN and PlexA1-DN could inhibit this association. In accordance with data concerning the biological response, neither SEMA3 DN receptor inhibited VEGF-A165-dependent association of Npn-1 with VEGF-R2 or VEGF-R2 tyrosine phosphorylation (Fig S2 d). Thus, SEMA3 DN receptors employed in this study specifically disrupt SEMA3 protein signalling, but do not inhibit either VEGF-A165 or PlGF2 signalling in ECs. SEMA3 proteins, Npn, and PlexA expression in cultured human ECs - Western blot analysis showed that cultured ECs synthesise SEMA3A, whose expression was enhanced by VEGF-A (Fig.S3 a; 2.4 fold increase after 24 hours). Synthesis of SEMA3A by unstimulated ECs is likely due to the fact that, when grown in vitro, ECs become 2 activated and express molecules usually induced in vivo by angiogenic factors16. Next, we tested whether SEMA3 genes other than SEMA3A are expressed by ECs. Semiquantitative RT-PCR analysis (Fig. S3 b) showed that ECs synthesise five out of six SEMA3 mRNAs and VEGF-A treatment increases transcription of all expressed SEMA3 genes except SEMA3F. Moreover, the mRNAs of both ligand binding and signal transducing subunits of SEMA3 receptor complexes, respectively represented by Npn (not shown and Refs. 11, 17) and PlexA (Fig. S3 b), were present in ECs. Supplementary methods In vivo angiogenic assays. Murine Matrigel plug 18 and CAM angiogenic assays19 were performed as previously described. Immunohistochemistry. Paraffin sections (5 m) were dewaxed by standard techniques. To improve staining, dewaxed sections were treated with antigen retrieval citra (BioGenex). Sections were incubated in 3% H2O2 for 10 min, and in 1% Triton X-100 for 10 min, and then blocked with antibody buffer (0.5% bovine serum albumin and serum) for 30 min at room temperature (RT). Primary antibodies were applied overnight at 4°C. Secondary antibodies were applied for 1 hr RT. Finally, sections were incubated in a streptavidin-peroxidase-complex (DAKO) according to manufacturer's instructions. Colour detection of immunoreactivity was achieved using 3'.3'-diaminobenzidine (DAKO). Sections were then counterstained in Mayer’s hemalum solution (MERK). Indirect immunofluorescence microscopy. Cells were plated onto FN-coated 0.95 cm2 glass coverslips, allowed to adhere for 20 min, fixed for 20 min RT in 3.7% paraformaldehyde (PAF), and then permeabilised for 2 min at 4°C with 0.1% Triton X100 in PBS. 3 Tissue samples were embedded in KILLIK (Bio-Optica) and snap frozen in precooled liquid isopentane. Four m serial sections were fixed for 20 min in freshly prepared solution of 3.7% PAF. Cells and tissue sections were sequentially incubated with the first primary antibody, revealed by a Cy3-tagged secondary antibody (Jackson Laboratories), and then with the second primary antibody, followed by the corresponding FITC-labelled secondary antibody (Jackson Laboratories). Specimens were observed with an inverted photomicroscope (model DM IRB HC; Leica Microsystems) equipped with mercury short arc epifluorescence lamp and appropriate combination of filters. The objective was immersion oil PL APO 63×/1.4. Images were captured using a cooled digital CCD Hamamatsu ORCA camera (Hamamatsu Photonics), digitally recorded and analysed with ImageProPlus 4.0 imaging software (Media Cybernetics). Cell motility assay and analysis of cell paths. 20,000 were plated onto 20 mm dish (Falcon) coated with 3 g/ml FN (Sigma), allowed to adhere in medium 199 for 1 h at 37 °C, and then observed with an inverted microscope equipped with thermostatic and CO2 controlled chamber. Images of motile ECs were captured with a 5 min time interval over 4 h using an ORCA camera (Hamamatsu Photonics). Images were then processed with DIAS software (Solltech). Cell motility data were displayed as a centroid plot showing the location of the geometrical centre of the cell as a function of time. Directional persistence was calculated by determining the ratio between the net path length and the total path length. Single cell trajectories were plotted using Excel software (Microsoft) and displayed in windrose graphs. Npn-1-DN construct. A two-step PCR protocol was used to generate a Npn-1 dominant negative (Npn-1 DN) construct where domains a1, a2, b1, and b2 were deleted and Npn-1 signal peptide was fused in frame with the c domain. Oligonucleotide primers were as 4 follows: i) cgccatggagagggggctgccgttg (F1) and cccattgggtgtcgtgggtccagcgaaagcccccagggcgagggc (R1) to amplify the signal sequence; ii) gctggacccacgacacccaatggg (F2) and ttctcaattcagatcctcttctgagatg (R2) to amplify the rat Npn-1 region spanning the c domain, transmembrane and cytoplasmic domains. The sequence on either side of the deleted region was amplified in the first step. The 5' end (underscored) of the internal reverse primer R1 is complimentary to the internal forward primer F2. Consequently, the two pieces of DNA amplified in the first round of PCR acted as the template for the second step of PCR, where they were left to anneal together at low temperature (45°C for 5 min.) and then amplified using the outermost primers F1 and R2. The C-terminus of Npn-1 DN was myc-tagged (italicised lettering in R2). The corresponding PCR product was TA-cloned into the pCR2.1TOPO (Invitrogen). RT-PCR analysis. Total RNA was isolated from ECs stimulated with 20 ng of VEGFA165 using TRI-REAGENT kit (Sigma) according to the manufacturer’s instructions, digested with DNAse, and subjected to reverse transcription (MuLV; Perkin Elmer). RT product was amplified in a PCR reaction with AmpliTaqGold (Perkin Elmer). Reactions were performed in a 9700 GeneAmp PCR System (Perkin Elmer) and calibrated on the exponential phase of amplification according to the following conditions: 10 min at 94°C, 30 sec at 94°C, 30 sec at annealing temperature (Tm), 30 sec at 72°C, and, finally, 7 min at 72°C for SEMA3A, 3B, 3C, 3E and 3F. The same conditions with a 45 sec of extension were used for SEMA3D, Plexin A1, A2, and A3 amplification. GAPDH was used as internal control. Primer sequences were as follows: SEMA3A fw 5’ ACTCACTGTTCAGACTTAC 3’ and re 5’ GAGCTGCATGAAGTCTCT 3’ (30 cycles, Tm 50°C). SEMA3B fw 5’ CAACTGGGCAGGGAAGGACAT 3’ and re 5’ CGTCTGGGTTCTCGCTCTCCG 3’ (37 cycles, Tm 60°C). SEMA3C fw 5’ GCAAAATGGCTGGCAAAGATCC 3’ and re 5’ CCCATGAAATCTATATACATTCC 3’ (37 cycles, Tm 60°C). SEMA3D fw 5’ 5 TTAGTCATGAAACTGCTG 3’ and re 5’ GCTCATCCAGGTCTCTGT 3’ (45 cycles, Tm 50°C). SEMA3E fw GTCTTATCCAAAGCATCCC 5’ 3’ CAACAGGCACACATGCAA (40 cycles, Tm 60°C). 3’ and re 5’ SEMA3F fw 5’ GCGCATGAAGTTGATCAC 3’ and re 5’ ACCAGTGGATGCCCTTCT 3’. PLEXIN A1, A2, A3 fw 5’ CCTCGAGA(G/A)CAAGAACCACCCCAAGCTGCT 3’ and re A1 5’ CCCTTCACCGGCACCTCAGGTGCATT AACACCTTCACTGGGATCTCTGGACTGTTC 3’, re 3’, A2 re A3 5’ 5’ CTTCACTGGGACCTGGGCGCTGCC 3’ (35 cycles, Tm 68°C). GAPDH fw 5’ ACCACAGTCCATGCCATCAC 3’ and re 5’ TCCACCACCCTGTTGCTGTA 3’. Intensity levels of PCR products were quantified using Phoretics 1D Standard (Abel Science-Warw SRL) and normalised for GAPDH levels. References 1. Kitsukawa, T. et al. Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron 19, 995-1005. (1997). 2. Chen, H., He, Z., Bagri, A. & Tessier-Lavigne, M. Semaphorin-neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron 21, 1283-90. (1998). 3. Giger, R. J. et al. Neuropilin-2 is a receptor for semaphorin IV: insight into the structural basis of receptor function and specificity. Neuron 21, 1079-92. (1998). 4. Takahashi, T., Nakamura, F., Jin, Z., Kalb, R. G. & Strittmatter, S. M. Semaphorins A and E act as antagonists of neuropilin-1 and agonists of neuropilin-2 receptors. Nat Neurosci 1, 487-93. (1998). 6 5. Raper, J. A. Semaphorins and their receptors in vertebrates and invertebrates. Curr Opin Neurobiol 10, 88-94. (2000). 6. Nakamura, F., Tanaka, M., Takahashi, T., Kalb, R. G. & Strittmatter, S. M. Neuropilin-1 extracellular domains mediate semaphorin D/III-induced growth cone collapse. Neuron 21, 1093-100. (1998). 7. Mamluk, R. et al. Neuropilin-1 binds vascular endothelial growth factor 165, placenta growth factor-2, and heparin via its b1b2 domain. J Biol Chem 277, 24818-25. (2002). 8. Gu, C. et al. Characterization of neuropilin-1 structural features that confer binding to semaphorin 3A and vascular endothelial growth factor 165. J Biol Chem 277, 18069-76. (2002). 9. Miao, H. Q. et al. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol 146, 233-42. (1999). 10. Migdal, M. et al. Neuropilin-1 is a placenta growth factor-2 receptor. J Biol Chem 273, 22272-8. (1998). 11. Gluzman-Poltorak, Z., Cohen, T., Herzog, Y. & Neufeld, G. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF165 [corrected]. J Biol Chem 275, 18040-5. (2000). 12. Rohm, B., Rahim, B., Kleiber, B., Hovatta, I. & Puschel, A. W. The semaphorin 3A receptor may directly regulate the activity of small GTPases. FEBS Lett 486, 68-72. (2000). 13. Tamagnone, L. & Comoglio, P. M. Signalling by semaphorin receptors: cell guidance and beyond. Trends Cell Biol 10, 377-83. (2000). 7 14. Tamagnone, L. et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99, 71-80. (1999). 15. Soker, S., Miao, H. Q., Nomi, M., Takashima, S. & Klagsbrun, M. VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J Cell Biochem 85, 357-68 (2002). 16. Garlanda, C. & Dejana, E. Heterogeneity of endothelial cells. Specific markers. Arterioscler Thromb Vasc Biol 17, 1193-202. (1997). 17. Soker, S., Takashima, S., Miao, H. Q., Neufeld, G. & Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform- specific receptor for vascular endothelial growth factor. Cell 92, 735-45. (1998). 18. Bussolino, F. et al. Platelet activating factor produced in vitro by Kaposi's sarcoma cells induces and sustains in vivo angiogenesis. J Clin Invest 96, 940-52. (1995). 19. Valdembri, D., Serini, G., Vacca, A., Ribatti, D. & Bussolino, F. In vivo activation of JAK2/STAT-3 pathway during angiogenesis induced by GM-CSF. Faseb J 16, 225-7. (2002). Supplementary Figure Legends Figure S1. Characterisation of Npn-1-DN. a, Structural features of wild type Npn-1 and Npn-1-DN. Npn-1-DN consists of the MAM/c, transmembrane, and cytoplasmic domain, the a1-a2 and b1-b2 domains of Npn-1 binding SEMA3 are missing and a Myc tag is present at its C-terminus. b, Western Blot analysis on U293 cells lysates transducted with Npn-1-DN. MAb directed against the Myc tag included in Npn-1-DN and polyclonal Ab recognising the cytoplasmic domain of Npn-1 stain the same band of at about 50kD. c, Western Blot of immunoprecipitated samples from WT or Npn-1-DN U293 cells cotransfected with different subunits of SEMA3 receptor complexes. Specific mAbs were 8 used directed against the VSV tag included in Npn-1, Npn-2, and plexins, or the HA tag included in Npn-1 and Npn-2. While in WT U293 the different subunits of SEMA3A receptor co-immunoprecipitate, the presence of Npn-1-DN impeded such interactions. Figure S2. Npn-1-DN and PlexA1-DN inhibit SEMA3A and SEMA3F, but not VEGFA165 and PlGF2 activity. a, Npn-1-DN and PlexA1-DN inhibit SEMA3A and SEMA3F activity. Cells were resuspended in the absence (Ctl) or the presence of SEMA3A or SEMA3F and allowed to migrate towards FN. b, c, Npn-1-DN and PlexA1-DN do not inhibit VEGF-A165 and PlGF2 activity. Cells were allowed to migrate towards 20 ng/ml VEGF-A165 or 30 ng/ml PlGF2 employing fibronectin (b) or vitronectin (c) as substrate. d, Npn-1-DN and PlexA1-DN do not inhibit VEGF-A165 dependent VEGF-R2 tyrosine phosphorylation and association with Npn-1. ECs were stimulated with 10 ng/ml VEGFA165 for 10 min. Cell lysates were immunoprecipitated with anti-VEGF-R2 and blotted with the indicated antibodies. Figure S3. SEMA3, Npn, and PlexA expression in cultured human ECs. a, VEGF regulates SEMA3A expression in ECs. Western Blot analysis of EC lysates reveals an increase (2.4 fold) in SEMA3A expression after 24 h VEGF-A stimulation. The goat polyclonal Ab N-17 recognising the N-terminus of SEMA3A specifically stains a protein of at about 100kD. b, ECs synthesise multiple SEMA3 mRNAs. Ethidium bromide– stained amplification products of semiquantitative RT-PCR with SEMA3A-3F, Plexin A1A3, and GAPDH–specific primer pairs are shown. mRNAs from cultured ECs stimulated with VEGF-A were used. Relative intensity levels of different PCR products were measured and normalised for GAPDH levels. mRNAs of SEMA3A, B, D, and E, but not SEMA3F are increased. ECs do not express SEMA3C mRNA even after VEGF stimulation. Transcripts of SEMA3A receptor subunits PlexinA1, A2, and A3 are present in ECs. Fold increases in red are higher than 1.5. 9