What is the rate of the reaction between iodine and acetone
advertisement

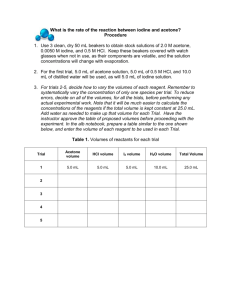
What is the rate of the reaction between iodine and acetone? The Question In this laboratory, the rate law describing the reaction between iodine and acetone in acidic solution will be determined experimentally using the initial rate method. To completely determine the rate law, the order of the reaction with respect to each reagent, and the rate constant at the reaction temperature must be determined. What is the rate of the reaction between iodine and acetone? Introduction In the study of reaction kinetics, the rate at which a chemical reaction occurs depends on several factors: (1) (2) (3) (4) The chemical properties of the reactants The concentrations of the reactants The reaction temperature The presence (if any) of catalysts. The rate of a reaction at a particular temperature is generally expressed in a mathematical equation of the form: Rate = k[A]m[B]n. In the rate law above, k is the rate constant; [A] and [B] are the molar concentrations of reagents A and B; and m and n are the orders of the reaction with respect to reagents A and B. The effects of temperature and catalysts are conveyed in the numerical value of the rate constant, k. The reaction being studied is that between iodine and acetone (as shown below), which occurs in aqueous solution in the presence of HCl: Although H+ does not appear as a reagent in this reaction, experimental observation shows that the reaction does not proceed unless H+ is present in the initial mixture. Therefore, the order of the reaction will be determined with respect to iodine, acetone and H+ concentrations. Iodine has a characteristic brown color in aqueous solution. The rate of the reaction can therefore be followed by measuring the time it takes for all the iodine initially present to react- the time it takes for the solution to convert from colored to clear and colorless. The experimental rate for each run is calculated as Rate = [Iodine]/t. The procedure here allows student groups to choose the specific concentrations of reagents to be used. To get reliable results, it is imperative that the scientific method be systematically employed. The scientific method assumes that variables are controlled, and that only one variable at a time is changed. Therefore, as the initial concentration of components is varied to determine the rate law, only one of the reagent concentrations should be changed between any pair of runs. Use of a systematic approach will allow easy determination of the effect of a specific change in concentration of a particular reactant on the rate. To simplify the calculations, consider changing the concentration of a reactant from one trial to the next by an integer factor (doubling, tripling, halving, etc.). This will make the subsequent mathematical analysis a lot easier than changing a concentration by a factor of 2.46, for example. The Objectives for this experiment are to: 1) design an initial rates procedure applying the scientific method 2) determine the order of the reaction with respect to iodine, acetone and HCl 3) calculate k, the rate constant, at lab temperature and 4) examine the effect of temperature on k. What is the rate of the reaction between iodine and acetone? Why Should I Care? Chemical Kinetics plays an important role in every process known to man. As shown in the abstract for this recent article from Geophysical Research letters, Geophysicists use the methods of chemical kinetics to understand the interactions between atmospheric and geological features on the earth. GEOPHYSICAL RESEARCH LETTERS, VOL. 32, L14815, doi:10.1029/2005GL023317, 2005 Initial uptake of ozone on Saharan dust at atmospheric relative humidities R. Y.-W. Chang Department of Chemistry, University of Toronto, Toronto, Ontario, Canada R. C. Sullivan Department of Chemistry, University of Toronto, Toronto, Ontario, Canada J. P. D. Abbatt Department of Chemistry, University of Toronto, Toronto, Ontario, Canada Abstract To better evaluate heterogeneous loss mechanisms for ozone in the atmosphere, the initial uptake kinetics of ozone on mineral dust surfaces was studied at room temperature under dry conditions and at elevated relative humidity. Ozone was added to a static absorption cell containing a glass tube coated with a thin film of Saharan dust collected from silt deposits on the Cape Verde Islands. Ozone concentrations were monitored by UV absorption at 254 nm, from which the reactive uptake coefficient in the first 10 s was calculated. An increase in initial ozone concentration from 2 × 1012 to 1 × 1014 cm−3 results in a decrease in the initial reactive uptake coefficient from 6 × 10−6 to 2 × 10−7, respectively, revealing a negative dependence of initial reactive uptake on initial ozone concentration. Simultaneously exposing the Saharan dust to ozone and water vapor at 50% and 75% relative humidity does not significantly change the initial reactive uptake coefficient. Received 25 April 2005; accepted 20 June 2005; published 23 July 2005. Read Full Article (file size: 208916 bytes) Cited by Citation: Chang, R. Y.-W., R. C. Sullivan, and J. P. D. Abbatt (2005), Initial uptake of ozone on Saharan dust at atmospheric relative humidities, Geophys. Res. Lett., 32, L14815, doi:10.1029/2005GL023317.