Cassidy Pomeroy-Carter Topic 1
advertisement

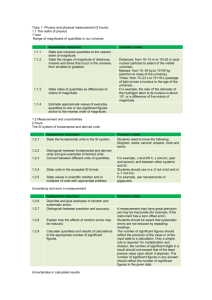
Physics and Physical Measurement 1.1.1 State and compare quantities to the nearest order of magnitude. Order of magnitude – power of ten (numbers are rounded up or down as appropriate) e.g. 2.03 x 103 → order of magnitude is 103 The value of using orders of magnitude is that comparisons can be easily made because working out the ratio between two powers of ten is just a matter of adding or subtracting whole numbers. e.g. ratio of diameter of an atom to diameter of a proton The order of magnitude of the diameter of an atom is 10-10 meters while the order of magnitude of the diameter of a proton is 10-15 meters. The ratio between them is thus 105 meters (the atom is 100,000 times larger). 1.1.2 State the ranges of magnitudes of distances, masses and times that occur in the universe, from the smallest to the greatest. For the IB, it is sufficient to know the following values: length ranges from the diameter of a proton (10-15 m) to the radius of the observable universe (1026 m) mass ranges from the mass of an electron (10-31 kg) to the total mass of the observable universe (1056 kg) time ranges from the passage of light across a nucleus (10-23 s) to the age of the Universe (1019 s) 1.1.3 State ratios of quantities as differences of orders of magnitude. See 1.1.1 and note that in the example given, the ratio 105 was found using the difference between the orders of magnitudes (105 = 10(-10 - -15)) 1.1.4 Estimate approximate values of everday quantities to one or two significant figures and/or to the nearest order of magnitude. This objective should be achieved through coursework. For a tutorial on significant figures, visit http://www.chem.sc.edu/faculty/morgan/resources/sigfigs/index.html 1.2.1 State the fundamental units in the SI system. Quantity mass length time electric current amount of substance temperature luminous intensity SI unit kilogram meter second ampere mole kelvin candela SI symbol kg m s A mol K cd It should be noted that the IB does not require the candela to be stated. 1.2.2 Distinguish between fundamental and derived units and give examples of derived units. Given the fundamental units, all other measurements can be expressed as different combinations of the fundamental units. The definition of the quantity being measured will often lead to the derived unit. e.g. Speed = distance / time units of speed = units of distance / units of time = meters / seconds (meters per second) = ms-1 SI derived unit newton (N) pascal (Pa) hertz (Hz) joule (J) watt (W) coulomb (C) volt (V) ohm (Ω) weber (Wb) SI base unit kg m s-2 Alternative SI units -1 kg m s-2 -1 s kg m2 s-2 N m -2 kg m2 s-3 As -1 kg m2 s-3 A kg m2 s-3 A-2 -1 kg m2 s-2 A gray (Gy) -1 kg s-2 A -1 s m2 s-2 sievert (Sv) m2 s-2 tesla (T) becquerel (Bq) N m-2 -1 Js -1 WA -1 VA Vs Wb m-2 J kg J kg -1 -1 1.2.3 Convert between different units of quantities. Converting between different units of quantities should be done using the factor label method (dimensional analysis). See the link below for a review: http://www.chem.tamu.edu/class/fyp/mathrev/mr-da.html 1.2.4 State units in the accepted SI format. Units in SI format should be expressed as they are in the charts above (i.e. meters per second should be expressed as ms-1 NOT as m / s. 1.2.5 State values in scientific notation and in multiples of units with appropriate prefixes. Prefixes can be used to denote powers of ten. Prefix T (tera) G (giga) M (mega) k (kilo) c (centi) m (milli) μ (micro) Value 1012 109 106 103 10-2 10-3 10-6 p (pico) 10-9 10-12 f (femto) 10-15 n (nano) For a review of scientific notation, see the following link: http://www.nyu.edu/pages/mathmol/textbook/scinot.html Remember that quantities in scientific notation should be expressed in standard form (only one number to the left of the decimal place!). 1.2.6 Describe and give examples of random and systematic errors. experimental error – there is a difference between the recorded value and the “perfect” or “correct” value random error – if you measure a quantity many times and get lots of slightly different readings (e.g. when measuring the bounce of a ball it is very difficult to get the same value every time even if the ball is doing the same thing) Possible sources: 1. the readability of the instrument 2. the observer being less than perfect 3. the effects of a change in the surroundings systematic error – occurs when something is wrong with the measuring device (e.g. measuring the bounce of the ball with a ruler with a broken end – will lead to zero error) Possible sources: 1. an instrument with zero error (to correct for zero error, the value should be subtracted from every reading 2. an instrument being wrongly calibrated 3. the observer being less than perfect in the same way in every measurement 1.2.7 Distinguish between precision and accuracy. Precision - if you measure the same thing many times and get the same value accuracy – if the measured value is close to expected James, Adilia, and Sarah Coutlee. “Notes on Uncertainty Analysis.” Chemistry 105 - Laboratory Manual. N.p., 3 July 2006. Web. 24 Mar. 2011. <http://www.wellesley.edu/Chemistry/ Chem105manual/Appendices/uncertainty_analysis.html>. 1.2.8 Explain how the effects of random errors may be reduced. To reduce random errors you can repeat your measurements. If the uncertainty is truly random, they will lie either side fo the true reading and the mean of these values will be close to the actual value. To reduce a systematic error you need to find out what is causing it and correct your measurements accordingly. A systematic error is not easy to spot by looking at the measurements, but is sometimes apparent when you look at the graph of your results or the final calculated value. 1.2.9 Calculate quantities and results of calculations to the appropriate number of significant figures. Significant digits should act as a guide to the amount of uncertainty. For example, a measurement of 35.476 kg implies an uncertainty of +/- 0.001 kg. When dealing with significant figures in calculations involving addition and subtraction, the result is rounded off to the last common digit occurring furthest to the right in all components. In other words, the result is rounded off so that it has the same number of decimal places as the measurement that has the fewest decimal places. When dealing with calculations involving multiplication and addition, the result should be rounded off so as to have the same number of significant figures as the component with the least number of significant digits. 1.2.10 State uncertainties as absolute, fractional and percentage uncertainties. Uncertainties can be expressed as absolute, fractional, or percentage uncertainties. For example, if a value is recorded as 2.0 +/- 0.6 g, this is a representation of absolute uncertainty. Dividing the absolute uncertainty by the recorded value gives the fractional uncertainty (0.6g / 2.0g). Multiplying the fractional uncertainty by 100 gives the value as a percentage uncertainty (2.0 g +/- 15%). 1.2.11 Determine the uncertainties in results. Whenever two or more quantities are multiplied or divided and they each have uncertainties, the overall uncertainty is approximately equal to the addition of the percentage uncertainties. This applies to powers as well. When a value is raised to a power, the uncertainty of the result is the percent uncertainty of the value multiplied by the power, which is the same as adding the percent uncertainty for each time the value is multiplied by itself. Whenever two or more quantities are added or subtracted and they each have uncertainties, the overall uncertainty is equal to the addition of the absolute uncertainties. 1.2.12 Identify uncertainties as error bars in graphs. Error bars are often used to represent uncertainties on graphs. The error bar represents the uncertainty range and so the line of best fit should pass through all of the rectangles created by the error bars. If this is not the case, the point may be considered an outlier or there may be a mistake in the relationship assumed to form the line of best fit (i.e. the best fit is a curve rather than a straight line). “Excel 2010: Error Bars.” Addictive Tips. N.p., n.d. Web. 24 Mar. 2011. <http://www.addictivetips.com/microsoft-office/excel-2010-error-bars/>. It should be noted that often the error bars are shown for either the independent or the dependent variable, especially if the uncertainty in the other variable is negligible. 1.2.13 State random uncertainty as an uncertainty range (+/-) and represent it graphically as an “error bar.” uncertainty range- applies to any experimental value; instead of giving just one value that implies perfection, a likely range for the measurement is given The determination of uncertainty can come from two sources: 1. Readability error Analogue scale +/- half the smallest scale division Digital scale +/- the smallest scale devision 2. Repeated measurements According to the IB Study Guide, when an average of measurements is found, the deviation of the smallest and largest measurements from this average should be found and the larger should be used as the uncertainty range. It can also be acceptable to take the range of the data and divide by two to find range uncertainty. 1.2.14 Determine uncertainties in the gradient and intercepts of a straight-line graph. If the gradient of the graph has been used to calculate a quantity, then the uncertainties of the points will give rise to an uncertainty in the gradient. Using the steepest and the shallowest lines possible (i.e. the lines that are still consistent with the error bars) the uncertainty range for the gradient is obtained (range between the steepest and the shallowest slope). If the intercept of the graph has been used to calculate a quantity, then the uncertainties of the points will give rise to an uncertainty in the intercept. Using the steepest and the shallowest lines possible (i.e. the lines that are still consistent with the error bars) we can obtain the uncertainty in the result (range between minimum and maximum possible value of intercept). For the determination of these uncertainties, error bars are needed only on the first and last data points. 1.3.1 Distinguish between vector and scalar quantities, and give examples of each. Vector – quantity with both magnitude and direction (e.g. displacement, velocity, acceleration, force, momentum, electric field strength, magnetic field strength, gravitational field strength) Vectors are usually indicated using a bold letter or a letter with an arrow above it. In diagrams, vectors are shown using arrows where the relative magnitudes of the vectors involved are shown by the relative length of the arrows and the direction of the vector is shown by the direction of the arrow. Scalar – quantity that only includes magnitude (e.g. distance, speed, mass, energy, temperature, potential or potential difference, density, area) 1.3.2 Determine the sum or difference of two vectors by a graphical method. The sum of two vectors can be determined in one of two ways graphically. The parallogram law of vectors is shown below: Delpierre, G R, and B T Sewell. “Addition of Vectors.” Electronic Science Tutor. N.p., n.d. Web. 24 Mar. 2011. <http://www.physchem.co.za/Vectors/Addition.htm>. The alternative method is adding the vectors “head to tail” as shown below: “Topic I: Vectors.” AP Physics C Website Notes and Plans for the Summer of 2010. N.p., n.d. Web. 24 Mar. 2011. <http://www.lnk2lrn.com/ap_physicssummer.html>. Subtracting vectors is merely adding the two vectors but switching the direction of the vector that is being subtracted. An example of “tail to tail” subtraction (which is really head to tail addition) is given: “The Basics of Vectors.” Knotebooks. N.p., n.d. Web. 24 Mar. 2011. knotebooks/3>. <http://knotebooks.com/ 1.3.3 Resolve vectors into perpendicular components along chosen axes. Vectors can be resolved into perpendicular components along chosen axes through the use of trignometry. A 5m thirty degree incline, for example, can be resolved into a horizontal and vertical component. The horizontal component is equal to 5m x cos 30, while the vertical component is equal to 5m x sin 30. “Right Triangle Trigonometry.” All About Circuits. N.p., n.d. Web. 24 Mar. 2011. <http://www.allaboutcircuits.com/vol_5/chpt_5/1.html>. The following trignometric identities should be useful. Sine x = O/H Cosine x = A/H Tangent x = O/A H2 = O2 + A2 Source: Kirk, Tim. “Physics and Physical Measurement.” IB Study Guide: Physics for the IB Diploma. Oxford: Oxford University Press, 2007. 1-10. Print.