Science work

.
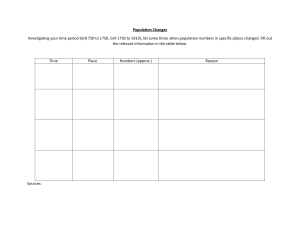
1 Explain some of the reasons why the list of elements was so small in 1750 (less than 20 known elements).
.
Some of the reasons that the list of elements in 1750 were so small that's because the technology was not that good back then and not able to pick up new elements
.
2 Research and outline some of the sources of electricity that were available before the invention of the electrochemical cell by Alessandro Volta.
.
.
3 The real breakthrough with organizing the elements into the periodic table came with the development of quantitative methods for measuring properties.
.
A) Explain the term ‘quantitative’.
.
.
Relating to, measuring, or measured by the quantity of something.
B) John Dalton developed away to measure the relative atomic mass of the different elements. Research and describe the meaning of the term
‘relative atomic mass’, using examples.
.
The mass of an atom of a chemical element expressed in atomic mass units
.
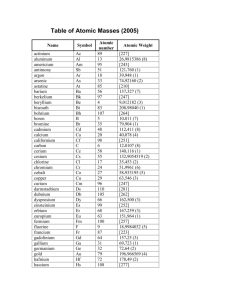
4 Examine the table showing the discovery of elements over time.
.
.
.
A) In small groups discuss reasons why some elements were easy to find and obtain, while others proved to be very difficult.
B) Construct two lists: • things that make elements easier to find and
purify • things that make elements difficult to find and purify
C) The element potassium is difficult to identify and collect, while the element carbon is easy. Propose possible reasons for this difference.
.
5 Choose one of the scientists mentioned in this chapter and research their background. Find out: a when they lived and died (or are they still alive?)