Distinguishing Phytophthora spp by sequencing
advertisement

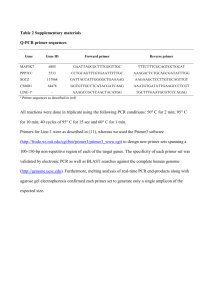
Identification of Phytophthora spp. By Direct Sequencing I. From Infected Plant Tissue or Water Samples Sample preparation Select up to 100 mg of diseased tissue and extract DNA using the DNEasy Plant Mini-kit. Freeze sample in liquid N and bead-beat (30 sec, 2500 rpm). Re-freeze and re-beat until sample is well-pulverized. Instructions for sample preparation from water samples are not yet available. Round I PCR Reaction Mixture (per 50 µl reaction) Nanopure water 14.5 µl MgCl2, 10 mM 7.5 µl Titanium Taq 10X buffer 5 µl Bovine serum albumin, 1 mg/ml* 5 µl dNTPs, 1.0 mM 5 µl Primer DC6, 5 µM 5 µl Primer ITS4, 5 µM 5 µl Titanium Taq 1 µl Round II PCR Reaction Mixture (per 50 µl reaction) Nanopure water 14.5 µl MgCl2, 10 mM 7.5 µl Titanium Taq 10X buffer 5 µl Bovine serum albumin, 1 mg/ml* 5 µl dNTPs, 1.0 mM 5 µl Primer ITS6, 5 µM 5 µl Primer ITS4, 5 µM 5 µl Titanium Taq 1 µl *Sigma Cat. No. A-7030, filter-sterilized. If PCR inhibition occurs or DNA yields are low, the PhytID site suggests using of 5 µl of 10 mg/ml BSA, but a white precipitation forms in the PCR tubes. Sample Round I: 2.0µl of DNA extract (or controls as noted below) Round II: 2.0µl of 1/500 dilution reaction mixture from Round I diluted in Nanopure water. Primers Prepare to a concentration of 5 µM (5 pmol/µl) in TNE buffer: DC6 5’-GAGGGACTTTTGGGTAATCA-3’ ITS6 5’-GAA GGT GAA GTC GTA ACA AGG-3’ ITS4 5’-TCC TCC GCT TAT TGA TAT GC-3‘ Controls Negative control: Positive control: 2 µl Nanopure water 2 µl genomic DNA from a known Phytophthora culture Cycling Protocol for Both PCR Rounds Name of Protocol: On SmartCycler: Phytophthora ITS On GeneAmp 9700: Phytophthora.its 1. 94ºC for 180 sec 2. 35 cycles of: 94ºC for 30 sec 55ºC for 30 sec 72ºC for 60 sec 3. 72ºC for 600 sec Duration of protocol: 98 minutes (SmartCycler) Approx. 115 min (GeneAmp 9700, max ramp rate) Expected Results The primer pair DC6/ITS4 produces 1.2 kb fragment. The DC6/ITS4 pair will reportedly amplify a 1.2 kb fragment only from Pythium, Phytophthora, and downy mildews (PhytID site, Bonants et al, 1997). The expected amplicon from Phytophthora species using the ITS6/ITS4 primer pair ranges from 862 bp to 941 bp. ITS6/ITS4 will amplify a band from a variety of organisms, but if the amplicon size is outside this range the organism is unlikely to be a Phytophthora, according to the PhytID website. Sequencing Amplicon First size the product by running 5 µl of the sample on a gel in 2.0% Nu-sieve 3:1 agarose in 0.5X TBE. Run the gel long enough so that the yellow dye runs off the gel and the blue dye has run 35-50% of the length of the gel. This allows you to check for the possible presence of two bands, which can result from mixed infection by Phytophthora and Pythium or two species of Phytophthora. Then run a preparative gel with 30-35 µl of reaction mixture from Round II in a 3.0% agarose gel in 0.5X TAE buffer. Excise band of interest and clean using the QiaQuick gel extraction kit or equivalent, paying special attention to instructions in the kit which relate to direct sequencing. Elute in 30 µl Buffer EB. If unreadable sequence results, this may suggest a need to clone, as mixed infections or insertion or deletion mutants in a strain that is heterozygous for the target gene of these primers would result in unreadable sequence. Notes There are at least three ITS-6 primers reported in the literature: the forward primer given above (from Cooke and Duncan, 1997); a reverse primer having the sequence 5’-AGG TAA TCC CGG TTG GTT TC-3’ and designed for detection of Oidium species (Matsuda et al., 2005); and a reverse primer having the sequence 5- TAC TGG GGC AAT CCC TGT TG -3) and which can be paired with ITS5 for amplifying the full ITS region of fungi (Bertini et al., 1999). II. From Mycelium in Culture Sample preparation On the PhytID website, the authors report that in most cases a tiny amount of aerial mycelium can be taken directly from a plate and subjected directly to PCR, obviating the DNA extraction step. However, the following extraction procedure has worked well in tests thus far. Grow suspect culture in V8 broth at room temperature in the dark for 48-72 hr. Tear off approximately 100 mg mycelium, rinse well in sterile Nanopure water, drain off excess water aseptically, and add to a bead-beater tube containing two 5-mm diameter beads. Hold on ice until ready for lysis. Freeze sample in liquid N and bead-beat once (30 sec, 2500 rpm). Then begin DNA extraction using the DNEasy Plant Mini kit. PCR Reaction Mixture (per 50 µl reaction) Nanopure water 14.5 µl MgCl2, 10 mM 7.5 µl Titanium Taq 10X buffer 5 µl Bovine serum albumin, 1 mg/ml* 5 µl dNTPs, 1.0 mM 5 µl Primer ITS6, 5 µM 5 µl Primer ITS4, 5 µM 5 µl Titanium Taq 1 µl *Sigma Cat. No. A-7030, filter-sterilized. If PCR inhibition occurs or DNA yields are low, the PhytID site suggests using of 5 µl of 10 mg/ml BSA, but a white precipitation forms in the PCR tubes. Sample 2.0µl of DNA extract (or controls as noted below) Primers Prepare to a concentration of 5 µM (5 pmol/µl) in TNE buffer: ITS6 5’-GAA GGT GAA GTC GTA ACA AGG-3’ ITS4 5’-TCC TCC GCT TAT TGA TAT GC-3‘ Controls Negative control: Positive control: Cycling Protocol 2 µl Nanopure water 2 µl genomic DNA from a known Phytophthora culture Name of Protocol: On SmartCycler: Phytophthora ITS On GeneAmp 9700: Phytophthora.its 1. 94ºC for 180 sec 2. 35 cycles of: 94ºC for 30 sec 55ºC for 30 sec 72ºC for 60 sec 3. 72ºC for 600 sec Duration of protocol: 98 minutes (SmartCycler) Approx. 100 min (GeneAmp 9700, max ramp rate) Expected Results The expected amplicon from Phytophthora species using the ITS6/ITS4 primer pair ranges from 862 bp to 941 bp. ITS6/ITS4 will amplify a band from a variety of organisms, but if the amplicon size is outside this range the organism is unlikely to be a Phytophthora, according to the PhytID website. Sequencing Amplicon Run a preparative gel with 30-35 µl of reaction mixture from Round II in a 3.0% agarose gel in 0.5X TAE buffer. Excise band of interest and clean using the QiaQuick gel extraction kit or equivalent, paying special attention to instructions in the kit which relate to direct sequencing. Elute in 30 µl Buffer EB. If unreadable sequence results, this may suggest a need to clone, as mixed infections or insertion or deletion mutants in a strain that is heterozygous for the target gene of these primers would result in unreadable sequence. References Bertini, L., Amicucci, A., Agostini, D., Polidori, E., Potenza, L., Guidi, C., Stocchi, V. 1999. A new pair of primers designed for ampli¢cation of the ITS region in Tuber species. FEMS Microbiology Letters 173:239-245. Bonants et al. 1997. Detection and identification of Phytophthora fragariae Hickman by the polymerase chain reaction. European Journal of Plant Pathology 103:345-355. Cooke, D. E. L., and Duncan, J. M. 1997. Phylogenetic analysis of Phytophthora species based on the ITS1 and ITS2 sequences of ribosomal DNA. Mycol. Res. 101:667-677. Matsuda, Y., Sameshima, T., Moriura, N., Inoue, K., Nonomura, T., Kakutani, K., Nishimura, H., Kusakari, S., Takamatsu, S., and Toyoda, H. 2005. Identification of individual powdery mildew fungi infecting leaves and direct detection of gene expression by single conidium polymerase chain reaction. Phytopathology 95:1137-1143. PhytID - Identification of Plant Pathogenic Phytophthora Species by ITS Fingerprinting. Online at http://www.phytid.org/ Document Control: February 16, 2016