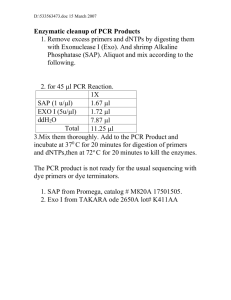
PCR Protocol for Screening
advertisement
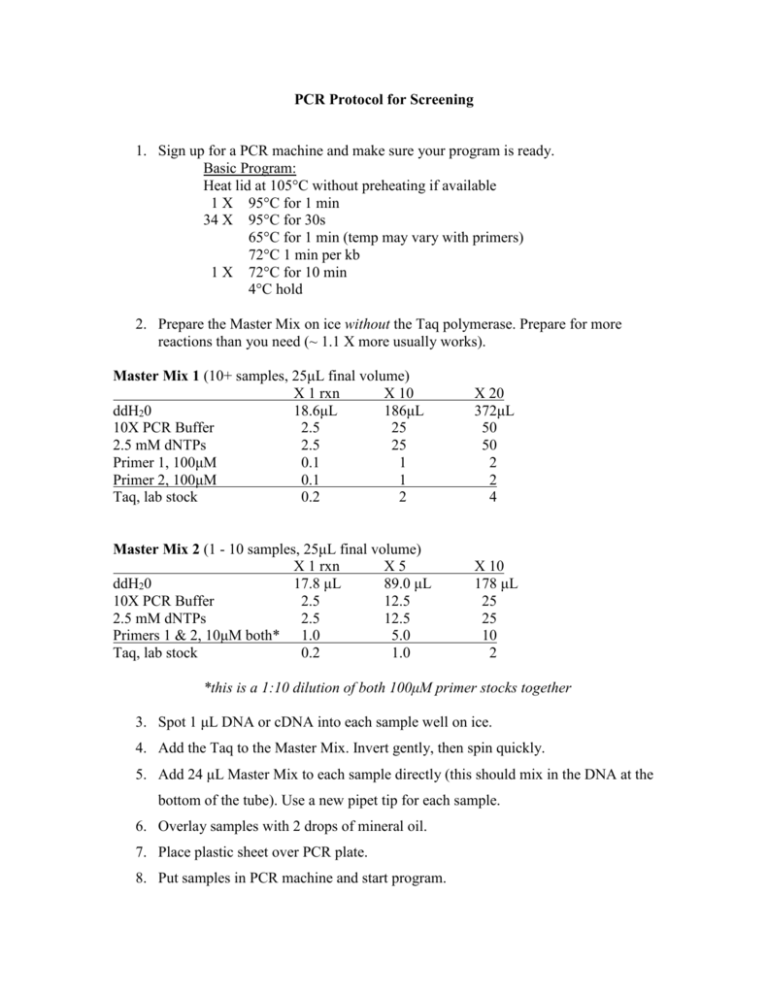
PCR Protocol for Screening 1. Sign up for a PCR machine and make sure your program is ready. Basic Program: Heat lid at 105°C without preheating if available 1 X 95°C for 1 min 34 X 95°C for 30s 65°C for 1 min (temp may vary with primers) 72°C 1 min per kb 1 X 72°C for 10 min 4°C hold 2. Prepare the Master Mix on ice without the Taq polymerase. Prepare for more reactions than you need (~ 1.1 X more usually works). Master Mix 1 (10+ samples, 25μL final volume) X 1 rxn X 10 ddH20 18.6μL 186μL 10X PCR Buffer 2.5 25 2.5 mM dNTPs 2.5 25 Primer 1, 100μM 0.1 1 Primer 2, 100μM 0.1 1 Taq, lab stock 0.2 2 X 20 372μL 50 50 2 2 4 Master Mix 2 (1 - 10 samples, 25μL final volume) X 1 rxn X5 ddH20 17.8 μL 89.0 μL 10X PCR Buffer 2.5 12.5 2.5 mM dNTPs 2.5 12.5 Primers 1 & 2, 10μM both* 1.0 5.0 Taq, lab stock 0.2 1.0 X 10 178 μL 25 25 10 2 *this is a 1:10 dilution of both 100μM primer stocks together 3. Spot 1 μL DNA or cDNA into each sample well on ice. 4. Add the Taq to the Master Mix. Invert gently, then spin quickly. 5. Add 24 μL Master Mix to each sample directly (this should mix in the DNA at the bottom of the tube). Use a new pipet tip for each sample. 6. Overlay samples with 2 drops of mineral oil. 7. Place plastic sheet over PCR plate. 8. Put samples in PCR machine and start program.