Protocol for in vitro ubiquitylation assay
advertisement

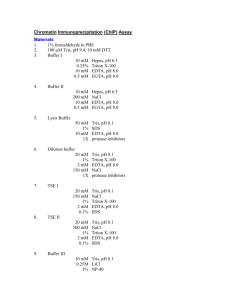
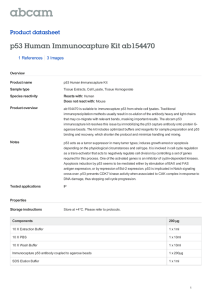
Protocol for in vitro ubiquitylation assay Feb, 2006 Yi Sheng Roger’s method (Cell 112 : 779): GST-Pirh2 0.5-1ug Rabbit E1 40 ng (Calbiochem) UBCH5B 100ng (Calbiochem) His-tagged ubiquitin 2ug (Sigma) 2 ul of BL-21 bacterial lysate ? In ubiquitylation buffer (50 mM Tris pH 7.4, 2mM ATP, 5mM MgCl2, 2mM DTT, 30 mM creatine phosphate, and 0.05mg/ml creatine phosphokinase to final volume of 30 ul. The reactions were incubated at 30 degree for 1.5 to 2 hrs. The reactions were stopped with 2 X SDS loading buffer, heated to 95 degree for 6 mins, and the proteins were separated on a 10% SDS-PAGE, ubiquitylated proteins were visualized by immunoblotting using a his antibody. For p53 ubiquitylation assays, s35 –labeled p53 protein was synthesized by in vitro transcription and translation using the TNT T7 coupled reticulocyte lysate system (promega). 2X104 cpm of in vitro translated p53 were added to GST-pirh2 (1-5 ug) and mixed on ice for at least for 1 hr to form GST-pirh2:p53 complexes. These complexes were then mixed with ubiquitination buffer and rabbit E1 (200ng), ubch2b (50 ng), and ub 5ug. The reactions were incubated at 30 degree for 1.5 to 2 hour, stopped with 2 x SDS loading buffer, resolved on 10% SDS-PAGE and analyzed by autography. Wei Gu’s method ( JBC 227 : 50607) Assay Mdm2 ub activity The in vitro ubiquitination assay was performed as described previous with some modifications. For a standard reaction, 10 ng of the bacteria produced GST-p53 or 5 ul of the purified acetylated p53 proteins from H1299 cells or 5 ul of the labeled substrates from a TNT reaction were mixed with other purified components, including E1 (12ng), E2 (GST-UBCH5c) (200ng), E3 GST-Mdm2 (500ng) and 2 ug of His-ubiquitin in a 20 ul reaction buffer (40 mM Tris , 5 mM MgCl2, 2 mM ATP, 2 mM DTT, pH 7.6). The reaction was stopped after 60 mins at 37 degree by addition of SDS gels or 4-12% gradient gels for either western blot analysis with a –p53 (DO-1) or autoradiography. Reagent: E1 (calbiochem) (10 ug) dilute to 25 ng per ul. (50mM Hepes pH7.6) E2 his-UBCH5b 1mg/ml in his elution buffer. Dilute 1:10 to 100ng/ul (50mM tris pH7.6) E3 GST-pirh2 1mg/ml Ub His-ub (sigma) 5 ul 1mg/ml 1. prepare 10x reaction buffer 500 mM Tris pH 7.6 50 mM MgCl2 20 mM DTT (fresh) 20 mM ATP (fresh) 2. Set up reaction (20ul) following the table: E3 GST-pirh2 full length E1 E2 Ub E3 buffer 1 E1 contr 2 5 2 2 2 E2 contr 2 5 2 2 3 E3 contr 2 2 5 2 4 E3 2 2 5 2 2 H2O 9 9 9 7 3. For screening E2, mix E1, ub, E3, buffer and H2O to make a master mix. 4. Aliquot 18ul of the master mix to each well. 5. Add 2 ul E2 (range from 100 ng to 400ng) to the 18 ul reaction mix to make the final volume to 20ul . 6. The reactions were incubated at 30 degree for 1.5 hour, stopped with 5 ul 5 x SDS loading buffer, resolved on 10% SDS-PAGE. 7. Followed by standard western-blot procedures. 8. Detect with anti-GST antibody to see the autoubiquitylation of GST-Pirh2. 9. To assay p53 ubiquitylatio by Pirh2, add 2 ul of 1mg/ml his-P53 full length into the reaction mix, follow the same procedure as above. Detect with anti-p53 antibody to see p53 ubiquitylation.